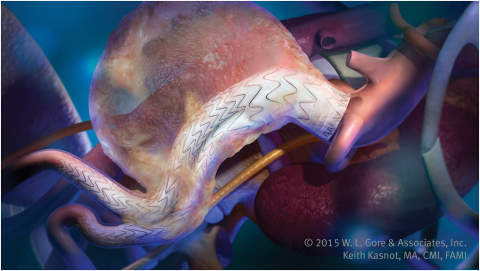
Gore Excluder is the next-generation endovascular aneurysm repair (EVAR) device for the treatment of various abdominal aortic aneurysms (AAA) in patients with challenging anatomies.
The patient is also the first recruitment in an investigator initiated post-market European registry called EXCeL (Excluder conformable real life). The principal investigator of registry is professor and Dr Marc van Sambeek.
Physicians can use the EVAR solution for the treatment of patients earlier excluded due to challenging proximal aortic necks.
Gore Excluder conformable AAA endoprosthesis with active control system secured CE mark approval for patients with proximal aortic neck angles of up to 90 degrees with a minimum 15 mm aortic neck length or in patients with proximal aortic neck angles of up to 60 degrees with a 10 mm minimum aortic neck length.
The procedure was carried out at Catharina Hospital in Eindhoven of Netherlands, under the guidance of Marc van Sambeek.
Sambeek said: “The conformability of the new GORE EXCLUDER Conformable Device in combination with the angulation control of the new delivery system will allow us to offer a less invasive, lower-risk alternative to open surgery to more AAA patients than ever before.”
The company will recruit 150 patients at the registry will in up to 11 European sites. Data from the trial will evaluate safety and treatment success of the device to treat infrarenal AAA in a range of anatomic presentations.
The device is also being assessed in a pivotal investigational US study, in which the first patient was already recruited.
Gore’s new delivery system is comprised of conformable stent graft, enhanced device positioning and optional angulation control.
Gore vascular business leader Eric Zacharias said: “With this first implant in Europe, we are continuing our commitment to offer the broadest and most technically advanced endovascular treatment capabilities to physicians and their patients.
“We are dedicated to developing technology that enables physicians to better treat challenging aortic anatomies. By collaborating with physicians, we’ve evolved our market-leading GORE EXCLUDER Device to enable more patients to be treated with EVAR.”





