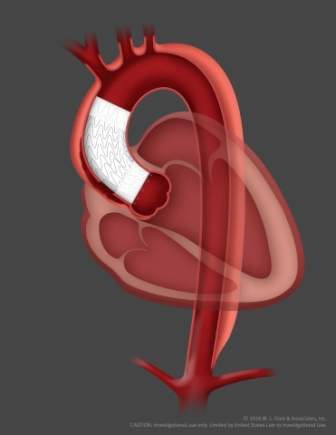
The GORE ascending stent graft features a delivery system that is designed to enable for accurate, controlled deployment in complex anatomies
As part of Arise study, the implant procedure was carried out by Anthony Estrera and Bruce Tjaden at the Memorial Hermann Medical Center in Houston of Texas.
The first multicenter and early feasibility Arise trial secured approval from the US Food and Drug Administration (FDA) to study the use of a minimally invasive device for the treatment of Type A dissection.
Initially, the study evaluates the use of Gore Tag thoracic branch endoprosthesis (aortic extender) in Type A dissection. It is also being assessed in its own pivotal study to evaluate safety and effectiveness in treating lesions of the aortic arch and descending thoracic aorta.
The Gore ascending stent graft is being used for the remainder of the study to enable investigators better understand about new technology in treating this condition.
The delivery system is also provided with an advanced angulation control that allows physicians to angulate the device to achieve orthogonal placement to the ascending aorta.
Type A aortic dissection is a tear in the lining of the ascending aorta, above the heart, which forms second channel of blood flow.
The study will assess how an endovascular stent graft delivered through catheter is being used to line the dissected portion of the ascending aorta as a less-invasive alternative to open surgical repair.
Gore vascular business leader Eric Zacharias said: “The development and clinical testing of this device are part of Gore’s ongoing mission to provide our physician partners with devices that solve unmet clinical needs that provide treatment options to their patients.”
The company’s aortic devices include conformable Gore Tag thoracic endoprosthesis to treat thoracic aneurysms, transections and Type B dissections and Gore Excluder AAA endoprosthesis to treat abdominal aortic aneurysms (AAA).
The Gore excluder iliac branch endoprosthesis (IBE) is said to be the first FDA approved off-the-shelf device for the endovascular treatment of common iliac artery aneurysms or aortoiliac aneurysms.




