The company is currently in the process of initiating their VAPOR 2 pivotal study in support of an FDA submission for U.S. market clearance
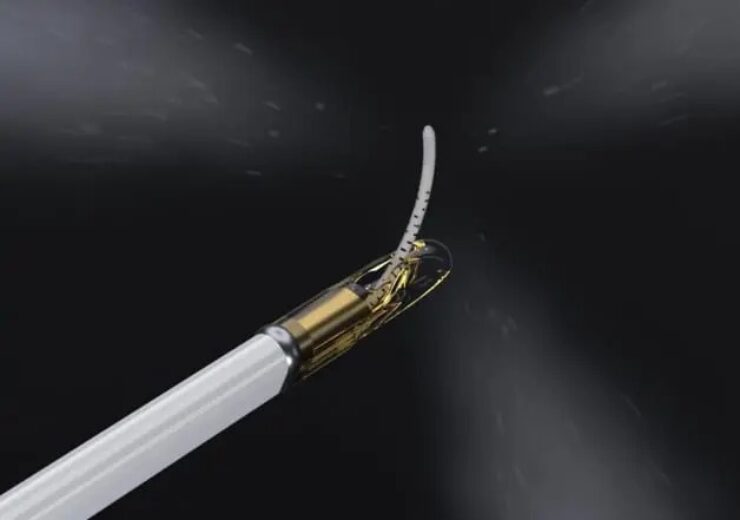
The Vanquish minimally invasive water vapor ablation therapy. (Credit: Francis Medical, Inc.)
Francis Medical, Inc., a privately held medical device company developing an innovative and proprietary water vapor ablation therapy for the treatment of prostate, kidney and bladder cancer, today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for its Vanquish minimally invasive water vapor ablation therapy.
Breakthrough Device Designation expedites the review of innovative technologies that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. To qualify for a Breakthrough Device Designation, a device technology must show that it has the potential to provide a more effective treatment than current standards of care. The goal of the program is to help patients have more timely access to these medical devices by expediting their development, assessment and review.
As the second most common cancer in U.S. men, the American Cancer Society estimates one in eight American men will be diagnosed with prostate cancer during their lifetime. Prostate cancer is a serious disease often treated with therapies that cause complications, such as urinary incontinence and erectile dysfunction. Francis Medical’s Vanquish water vapor technology applies the thermal energy stored in a few drops of sterile water to deliver targeted treatments to the cancerous tissue through a simple transurethral procedure. The therapy is designed to ablate cancer cells while protecting surrounding structures, lessening the likelihood of life-altering side effects common with other prostate cancer treatments.
“The goal of Francis Medical is to become the first line therapy of choice for patients with prostate cancer,” said Michael Kujak, Francis Medical president and chief executive officer. “We are excited that the FDA has recognized the potential of our technology to be a breakthrough for patients who today face the difficult choice between addressing their cancer and avoiding the debilitating morbidities often associated with current standards of care.”
The company is currently in the process of initiating their VAPOR 2 pivotal study in support of an FDA submission for U.S. market clearance. The VAPOR 2 study will treat 235 patients with localized, intermediate-risk prostate cancer at up to 30 centers in the U.S. Further information on the VAPOR 2 clinical study can be found at clinicaltrials.gov (NCT05683691).
Source: Company Press Release
