GPX Embolic Device leverages the firm’s core GPX technology to occlude blood vessels regardless of the patient’s coagulation status
GPX-Clear Embolic Device in-vivo research. (Credit: Fluidx Medical Technology.)
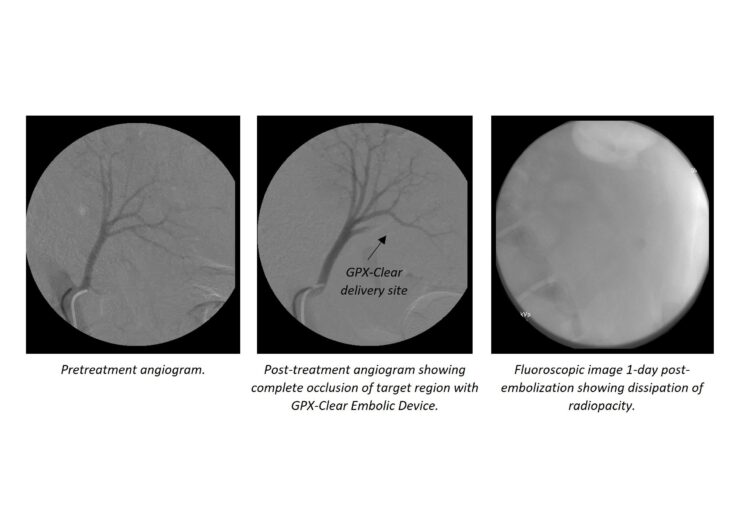
Fluidx Medical Technology, the developer of advanced embolic material GPX, has announced positive visibility results for its GPX-Clear Embolic Device from an in-vivo study.
The in-vivo research used the company’s base GPX technology, incorporated with an intermediate term radiopacity agent.
The GPX-Clear Embolic Device leverages the core GPX technology, along with a non-artifact inducing radiopacity agent, trapped within the polymer matrix.
The radiopacity agent is designed to provide superior visibility of the embolic device during and after the embolic delivery.
By dissolving within 24 hours after the delivery, the radiopacity agent facilitates unobstructed visibility of the treated area, said the medical technology company.
University of Utah, Salt Lake City, Utah, interventional oncologist Ryan O’Hara said: “Our initial in-vivo work with GPX-Clear looks very promising.
“It has the advantages of the baseline GPX product around ease of use, minimal preparation and compatibility with standard microcatheters, with the added benefit of radiopacity that dissipates within the first 24 hours.
“There are many instances, particularly in oncology, in which clear visibility of the treated region post-embolization is critical and can be obscured by radiopacity agents. This is an exciting future product in the GPX line-up and the first product of its kind.”
Fluidx Medical said that its GPX Embolic Device is designed to occlude blood vessels regardless of the patient’s coagulation status.
The device comes as a ready-to-use package in a syringe, to facilitate simple preparation and controlled embolic delivery.
It can be prepped by the clinician on the patient’s tableside, within about 30 seconds, and does not require DMSO precipitation.
Also, it can be delivered through standard microcatheters, eliminating the need for complex mixing systems or special delivery catheters.
Fluidx Medical stated that its GPX Embolic Device is currently under development, and has not been approved in any market, except for investigational use in New Zealand.
GPX-Clear R&D director Danny Smith said: “The Fluidx team is pleased with these initial results and excited to be building out the GPX Embolic Device platform.
“We continue to identify clinical needs in the market and create new devices that meet those needs and improve patient care.”
