The first patient received treatment using Monarch platform, as part of a clinical study at UCI Health, for a robotically assisted electromagnetic (EM)-guided percutaneous access and mini-percutaneous nephrolithotomy (PCNL) procedure
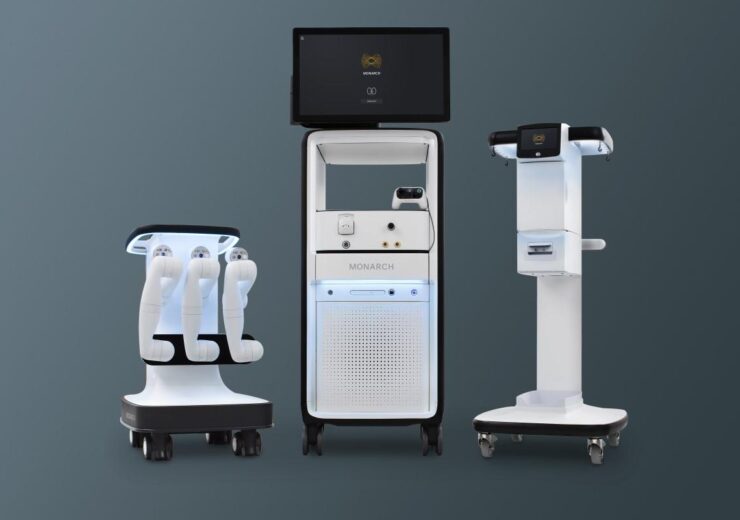
The Monarch platform for robot-assisted urology procedures. (Credit: Medical Device Business Services, Inc.)
Ethicon, a company of Johnson & Johnson MedTech, has announced the first robotic-assisted removal of kidney stones using its Monarch platform for Urology.
The first patient received the Monarch platform treatment, as part of a clinical study conducted at UCI Health, the clinical enterprise of the University of California, Irvine (UCI).
In the clinical trial, the platform was used to perform robotically assisted electromagnetic (EM)-guided percutaneous access and mini-percutaneous nephrolithotomy (PCNL) procedure.
It was conducted in collaboration with co-investigator Dr. Mihir Desai from the University of Southern California (USC).
Dr. Desai said: “The prevalence of kidney stones remains high, and many urologists seek a new treatment option that reduces overall retreatment and complication rates. In patients who require treatment through surgery, close to one in two will require retreatment within five years.”
Monarch is a flexible robotic platform intended for use in bronchoscopy and urology procedures, which received the US Food and Drug Administration (FDA) in April last year.
The platform is designed to enable urologists navigate through the kidney using robotic-assistance designed for access and control using a handheld controller.
It provides the urologists with a single platform that supports both ureteroscopic and percutaneous nephrolithotomy (PCNL) procedures, said Ethicon.
Monarch uses a novel EM targeting platform to help urologists gain more precise access to the kidney and to achieve overall better outcomes in a single procedure.
According to the company, ureteroscopic procedures are the most common surgical stone procedures worldwide, which become highly challenging with the increase in stone size.
PCNL procedures are associated with several barriers and Monarch platform allows urologists to overcome the obstacles using unique and minimally invasive technology.
Furthermore, the platform provides 80% less radiation exposure, enhanced accuracy and consistency, compared to standard fluoroscopic guided access techniques, said Ethicon.
UCI School of Medicine department of urology chair and UCI Health kidney stone & kidney disease services director Jaime Landman said: “This clinical study is the first in the world to research and demonstrate the potential for improved navigation, access, clearance, and control in mini-PCNL procedures using the MONARCH Platform for Urology.
“In addition to potentially helping urologists achieve stone-free patients in a single procedure, this approach could help reduce the need for retreatment after kidney stone removal and decrease risks and complication rates.
“After years of work, we are thrilled to be a part of this first clinical series which introduces a new treatment to improve outcomes for patients in need.”
