CMFlex is a 3D-printed synthetic bone graft used for various bony defects in oral and maxillofacial surgical applications
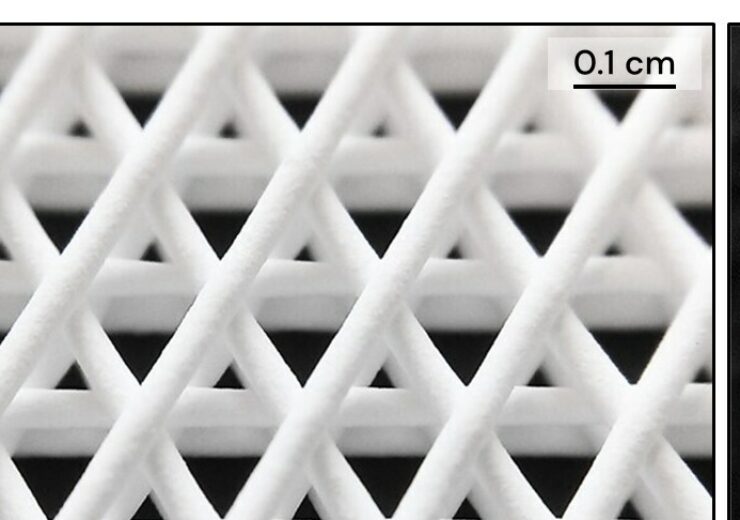
CMFlex has an intentionally engineered three-dimensional architecture. (Credit: PRNewswire/Dimension Inx)
Dimension Inx, a biomaterials platform company, today announced that CMFlex™, the first 3D-printed regenerative bone graft product with FDA approval, has been successfully used in its first clinical cases.
The first two cases were performed by Dr. Derek Steinbacher, Director of West River Surgery Center, Former Professor of Plastic surgery and Chief of Oral and Maxillofacial surgery at Yale New Haven Health and Dr. Brian Farrell, DDS, MD, of the Carolinas Center for Oral & Facial Surgery. Procedures performed included a mandibular angle augmentation (surgery of the lower jaw) and maxillary segmental osteotomy (surgery of the upper jaw).
CMFlex is a 3D-printed synthetic bone graft used for various bony defects in oral and maxillofacial surgical applications. CMFlex utilizes a chemistry comprised primarily of hydroxyapatite particles combined with biodegradable polylactide-co-glycolide (PLG) polymer. Both materials have an extensive history demonstrating biocompatibility and clinical utility. Dimension Inx combines these base materials into a proprietary, microstructurally porous composite material, Hyperelastic Bone®, as first published in Science Translational Medicine in 2016. This unique, easily deployable bone repair material is then 3D-printed into CMFlex. The resulting product is one that surgeons can size for each patient and is uniquely capable of absorbing fluid, which enables it to control bleeding during surgery while assisting the bone remodeling process once implanted.
“These first cases are not only indicative of a new generation of biomaterials, but also highlight our technology platform’s unique capability to rapidly create biomaterials that direct cell behavior to restore tissue and organ function. It is a proud moment for us to be able to demonstrate the value of therapeutics derived from integrating novel biomaterial design and 3D-printing approaches,” stated Dr. Adam Jakus, CTO, Head of Technology Strategy, and Dimension Inx co-founder.
Dr. Ramille Shah, CSO, Head of R&D, and co-founder of Dimension Inx, further expounded, “We are thrilled to share this solution with the surgical community. CMFlex is a product that represents our unique approach to restoring functionality in the body: it’s a dynamic collaboration between biology, material composition, microstructure, and macroarchitecture. We’re excited by the interest we’ve already received from surgeons who recognize the importance of a ready-to-use solution with great handling characteristics and the ability to cut and shape the graft to match the defect site.”
Clinicians speak to the benefits of CMFlex: “CMFlex offers a novel ready-to-use bone graft solution that boasts excellent handling characteristics, and the fact it can also be cut and trimmed to easily match the defect site made it an exceptional product to work with”, stated Dr. Steinbacher.
Dr. Farrell expressed similar remarks, further emphasizing that “the material was easy to contour, and I anticipate ongoing utilization to monitor its performance over time”.
CMFlex has also been used in several socket preservation surgeries for future dental implant placement. “I have been thoroughly impressed with the product’s ability to promote hemostasis, which simplified the implantation process”, shared Dr. Robert Bosack, DDS, Oral, Maxillofacial & Dental Implant Surgery.
Dimension Inx received FDA clearance of CMFlex in December of 2022. CMFlex is currently available to a limited number of key surgeons with a broader release to follow later in 2024.
Source: Company Press Release
