The CurvaFix IM Implant is intended to address multiple pelvic fracture fixation challenges, including dysmorphic sacra and curved superior rami
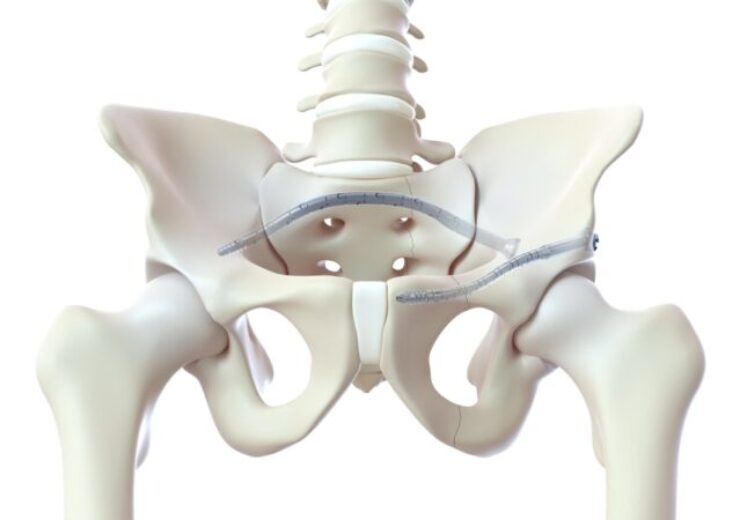
The new CurvaFix IM Implant. (Credit: Business Wire)
CurvaFix has launched its smaller-diameter, 7.5mm CurvaFix IM Implant for the simplification of surgery and providing strong, stable fixation in small-boned patients.
The new intramedullary (IM) device has been implanted already in more than two dozen patients, claimed the US-based medical devices maker.
The CurvaFix IM Implant is intended to address various pelvic fracture fixation challenges, including dysmorphic sacra and curved superior rami.
CurvaFix said that it will exhibit the implant at the upcoming American Academy of Orthopedic Surgeons (AAOS) 2023 in Las Vegas. The firm will also present the 9.5mm CurvaFix Implant, introduced in late 2021, and provide updates from its recent US cases.
CurvaFix CEO Steve Dimmer said: “The new 7.5mm device is designed to simplify surgery and provide strong, stable, curved fixation in smaller patients.
“Additionally, our novel device has been shown to offer many geriatric patients immediate pain relief and early mobility, which is critically important in older patients where mobility is such a key to life.”
The medical device maker reported that so far more than 175 patients have had CurvaFix treatment, including over 100 elderly individuals and/or those with fragility pelvic fractures (FFP).
The use of over 240 CurvaFix Implants by 35 US doctors has shown that a longer, wider, curved implant can quickly alleviate pain and enable early mobility in a variety of patients with a minimally invasive technique, said the company.
The implanted patient population showed surgical utility and potential advantages in a range of pelvic injuries and conditions.
The IM implant secured 510(k) clearance from the US Food & Drug Administration (FDA) in November last year.
