The HeartLight X3 System is a third generation AFib ablation technology designed based on the advanced features of the HeartLight Endoscopic Ablation System
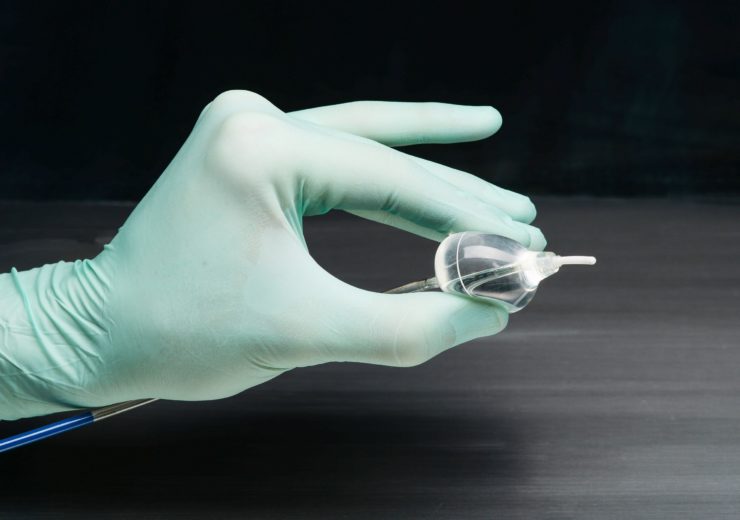
The HeartLight Excalibur Balloon used with the HeartLight X3 System. (Credit: CardioFocus, Inc.)
US-based medical device firm CardioFocus has submitted a pre-market approval (PMA) supplement to the US Food and Drug Administration (FDA) for the HeartLight X3 endoscopic ablation system to treat atrial fibrillation (AFib).
The latest supplement follows the earlier approved PMA of the HeartLight endoscopic system.
The system will be commercially launched in the US following the PMA supplement approval.
Details of the HeartLight X3 System
The HeartLight X3 system is a third generation AFib ablation technology designed based on the advanced features of the HeartLight endoscopic ablation system.
The HeartLight endoscopic ablation system conducts pulmonary vein isolation (PVI) using laser energy to create lines of scar tissue to block the abnormal electrical pathways that cause AFib.
CardioFocus stated that using direct tissue visualisation, titratable laser energy, and compliant balloon technology, the HeartLight X3 system’s RAPID mode creates uninterrupted, high-speed, circumferential lesion under direct control of the physician and reduces procedure time.
The system steadily achieved very fast PVI within three minutes for a single vein in the pivotal confirmatory assessment of 60 patients.
CardioFocus CEO Burke Barrett said: “The excitement surrounding the introduction of HeartLight X3 into the European market has been palpable.
“We have treated nearly 500 patients with the technology and the ability to deploy rapid, continuous laser energy circumferentially and outside of the pulmonary vein is compelling.
“The submission of this PMA supplement marks a tremendous milestone as we prepare to bring this breakthrough product to the U.S. Based on our initial interactions with FDA, we anticipate launching HeartLight X3 into the U.S. market this year.”
The HeartLight X3 system secured the European CE Mark approval in March 2019 and is approved for use only in Europe.
In May 2019, CardioFocus completed £43.4m round of financing to advance the ablation treatment for AFib.
The firm had stated that its existing syndicate of institutional investors participated the financing round and included senior debt financing from Kennedy Lewis Investment Management.
