Designed for use with the company’s Vercise Genus Deep Brain Stimulation (DBS) Systems, the Vercise Neural Navigator 5 Software provides data that enables clinicians to effectively plan the treatment for people with Parkinson’s disease or essential tremor
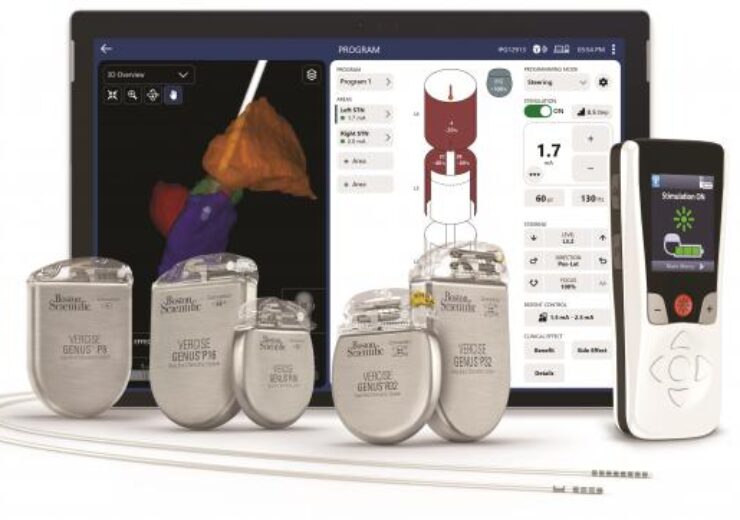
The Vercise Neural Navigator 5 with StimView XT technology. (Credit: Boston Scientific)
US-based medical devices manufacturer Boston Scientific has received the US Food and Drug Administration (FDA) approval for its Vercise Neural Navigator 5 Software.
Boston Scientific has designed the Vercise Neural Navigator 5 Software for use with its Vercise Genus Deep Brain Stimulation (DBS) Systems.
The Vercise Genus DBS System works by delivering targeted electrical stimulation through surgically-implanted leads in the brain, connected to an implantable pulse generator (IPG).
The software, when used with Vercise Genus DBS systems, will provide data that enables clinicians effectively plan the treatment for people with Parkinson’s disease or essential tremor.
Furthermore, Vercise Neural Navigator 5 Software facilitates better management of the evolving needs of individual patients at any stage of their condition, said the medical device maker.
Boston Scientific neuromodulation president Jim Cassidy said: “Developing meaningful tools to help physicians provide personalised treatments for their patients delivers on our promise to advance our technologies for people living with neurological conditions.
“Providing effective DBS therapy is complex and can be time-consuming. This software will help streamline the process and allow for more doctor-patient interaction time.”
Boston Scientific has developed the Vercise Neural Navigator 5 Software in collaboration with Germany-based medical technology company Brainlab.
The software, which features the STIMVIEW XT Technology, is the latest addition to the company’s integrated portfolio of image guided programming solutions for its DBS Systems.
It comes with an enhanced user interface to display patient data in a simplified format and provides clinicians with access to advanced settings for increased therapy delivery.
The tools showed a reduction in programming time by 56% and offered real-time visualisation and stimulation of each person’s unique brain anatomy, said the medical device company.
Wake Forest School of Medicine neurology and neurosurgery professor, and Atrium Health Wake Forest Baptist DBS programme medical director Mustafa Saad Siddiqui said: “The ability to see the precise placement of DBS Systems enables us to target therapy to meet individual needs.
“The new features in the Vercise Neural Navigator 5 are expected to help further reduce the time needed to adjust stimulation and minimise potential side effects, allowing us to optimize treatment benefits for each patient.”
