The Passio pump is attached to an implanted Passio catheter using the disposable collection kit and is activated to begin the evacuation of fluid into the collection bag
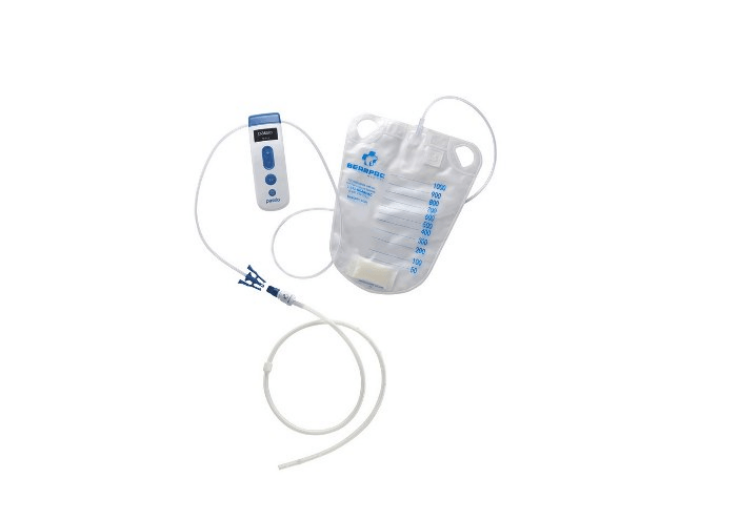
The Passio pump drainage system and Passio catheter. (Credit: PRNewswire / Bearpac Medical, LLC)
Bearpac Medical, LLC is pleased to announce that they have launched a new FDA 510(k) cleared device intended to treat patients with malignant and other recurrent pleural effusions.
For the first time, a Class II electronic handheld pump will be available to clinicians for a more controlled delivery of therapy for patients experiencing pleural effusions.
The Passio Pump Drainage System consists of the Passio Catheter, a Handheld Control Unit (pump) and a Disposable Collection Kit, which includes a redressing kit, for drainage of recurrent and symptomatic pleural effusion.
The Passio pump is attached to an implanted Passio catheter using the disposable collection kit and is activated to begin the evacuation of fluid into the collection bag.The Passio catheter is exclusively designed for use with the Passio collection system.
Passio provides electronic flow control throughout the therapy at lower vacuum pressures than competitive devices on the market today.
“Pleural effusion drainage devices haven’t changed in decades. Bearpac’s strategy of combining technology with a user friendly design allow the caregiver and/or patient the ultimate control over the therapy. Our ultimate goal is to make this stage of their treatment less painful,” said Jay Zimmerman, President of Bearpac Medical.
Source: Company Press Release
