The mixed reality-based VSI HoloMedicine provides an almost x-ray vision perspective to surgeons in surgical planning processes by using 3D holographic technology
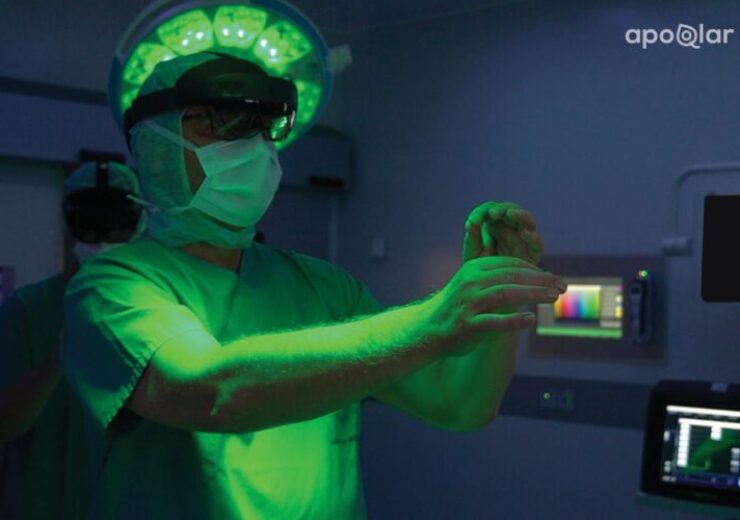
Surgeon visualising 3D holograms in medical mixed reality. (Credit: PR Newswire/apoQlar GmbH)Surgeon visualising 3D holograms in medical mixed reality. (Credit: PR Newswire/apoQlar GmbH)
Germany-based medical metaverse company apoQlar has secured the US Food and Drug Administration (FDA) 510(k) Class II clearance for its mixed reality-based VSI HoloMedicine software device.
According to the firm, the surgical planning platform enables surgeons to plan complex procedures by using immersive 3D holographic technology.
VSI HoloMedicine is said to provide surgeons with close to an x-ray vision perspective during surgical planning processes.
Using the 3D holographic technology, doctors from all medical specialties can visualise medical data inside or outside of the operating room and plan surgeries in 3D, apoQlar said. This is said to give a third dimension to surgical planning, patient education, remote consultation, and medical training.
Surgeons can create interactive 3D holograms from flat CT, MRI, Angio CT, CBCT, PET, and SPECT sources using Microsoft’s HoloLens 2, which is a mixed reality head-mounted display (HMD).
ApoQlar co-founder and CEO Sirko Pelzl said: “With mixed reality, we are no longer bound to physical objects in a physical world. We can leverage digital objects and services on top of the real world for equal or greater utility and usually at a fraction of the cost.
“Mixed Reality is a completely new way for people, and in our case surgeons, physicians and technologists, to continue to experience the real world around them but with an entire virtual layer placed on top.”
With the FDA clearance, the US becomes the 30th nation where apoQlar has secured medical certification. Its device already has CE Class I and HSA Class A medical certifications for clinical use in 30 countries.
The VSI HoloMedicine device is expected to be available in the US in Q2 2023 through apoQlar’s subsidiary in Miami, Florida.
Currently, the medical metaverse firm will conduct its first-ever funding effort to raise a Series A round in order to scale VSI Holomedicine as the base for modern surgical care.
