Brainance MD is a web-based and all-in-one software that is designed for diffusion tensor imaging (DTI), dynamic susceptibility contrast (DSC) perfusion, and functional MRI (fMRI)
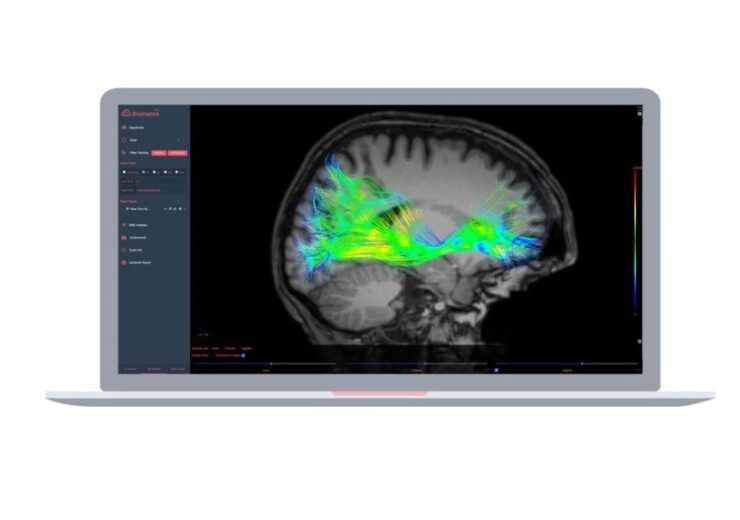
Advantis Medical Imaging receives FDA clearance for its pure web-based and all-in-one neuroimaging platform. (Credit: PRNewswire / Advantis Medical Imaging)
Advantis Medical Imaging has secured 510(k) clearance from the US Food and Drug Administration (FDA) for its neuroimaging platform Brainance MD to analyse brain magnetic resonance imaging (MRI) exams.
Brainance MD, which is designed to access from a web browser, allows to display, process, and analyse brain MR images through a unified user interface.
The analysis software can be used for diffusion tensor imaging (DTI), dynamic susceptibility contrast (DSC) perfusion, and functional MRI (fMRI).
Designed to provide as a Software as a Service (SaaS), the fully DICOM compliant application delivers analysis and visualisation capabilities of dynamic MRI data of the brain.
It enables to present the results in a clinically useful context, as well as supports clinicians during the diagnostic workflow of brain MRI exams.
Advantis Medical Imaging CEO and CSO Zoi Giavri said: “Advanced brain MRI processing techniques which were previously regarded as more research-oriented due to their scientific complexity and time-consuming manual processing needs are now available to clinicians in an all-in-one, automated and highly intuitive software.
“Our solution eliminates any unnecessary manual work, embraces remote access and collaborative exam review, enhances standardisation and reduces operating overhead for clinical organizations through its zero-footprint nature and optimized processing pipelines.”
The neuroimaging platform already secured CE mark approval in Europe, and is used by multiple clinical organisations in their daily clinical brain MRI processing workflows.
Established in 2016, Advantis Medical Imaging has offices in Eindhoven of Netherlands and Athens of Greece.
