The first study to test a catheter-based, non-surgical treatment for patients with severe tricuspid regurgitation has been launched by Abbott
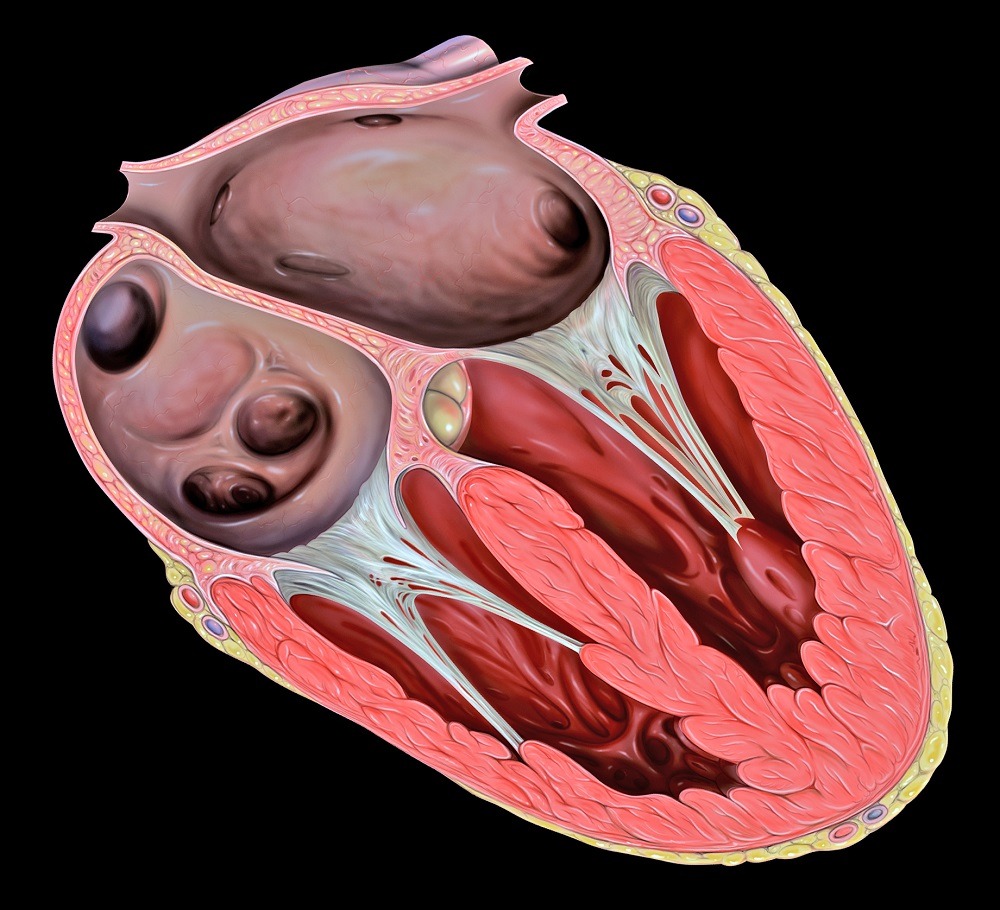
Despite the prevalence of tricuspid valve disease, it is often referred to as the 'forgotten heart valve' as treatment options are so limited (Credit: Wikimedia)
The safety and effectiveness of TriClip, a new leaky valve repair system developed by Abbott, will be tested in a world-first clinical trial launched yesterday.
It will be the first Investigational Device Exemption trial in the US to evaluate a catheter-based, non-surgical treatment for patients with severe tricuspid regurgitation (TR).
The condition results to blood leaking backwards across the valve, which forces the heart to work harder and can lead to heart failure if left untreated.
Dr David Adams, chairman of the department of cardiovascular surgery at the Icahn School of Medicine at Mount Sinai, and co-primary investigator in the trial, said: “Patients with symptomatic tricuspid regurgitation are often at an increased risk for conventional surgery.
“As a result, many are not referred for intervention.
“The opportunity to assess how we can better treat these patients with a minimally invasive approach is critical and we’re excited about the potential for this therapy in improving the quality of life for these patients.”
How will Abbott’s new TriClip system be tested?
The prevalence of moderate-to-severe TR in the US has been reported at more than 1. 6 million, yet only 8,000 operations are performed annually in the US.
There are currently no approved non-surgical, minimally invasive treatments for people with severe TR.
Known as TRILUMINATE, the clinical trial has had its first enrolments at the Abbott Northwestern Hospital in Minneapolis, during which the TriClip device was accepted for national reimbursement consideration through a process called Parallel Review.
The Parallel Review initiative is a collaborative effort that is intended to reduce the time between FDA approval and Medicare coverage decisions, thereby “facilitating earlier access to innovative medical technologies for Medicare beneficiaries.”
The multi-centre study will evaluate the performance of the clip-based technology in approximately 75 symptomatic patients at 25 sites across the US and Europe.
Chief medical officer of Abbott’s structural heart business Dr Neil Moat said: “While we’ve made substantial progress on a number of fronts for challenging structural heart conditions, tricuspid regurgitation impacts far too many patients worldwide, and physicians are limited by a lack of meaningful therapy alternatives to surgery.
“Early results with our TriClip repair system have been encouraging and we’re excited to continue driving innovation that we believe will benefit more patients in the future.”
The TriClip valve repair system is built on Abbott’s MitraClip system, which is claimed to be the only transcatheter mitral valve therapy with proven safety and survival, and durable clinical outcomes. It has been commercially available in the US since 2013.
