Sky Medical Technology's head of product research and development explains the journey geko has taken in pursuit of DVT prevention, as well as being a tool to combat other types of disease
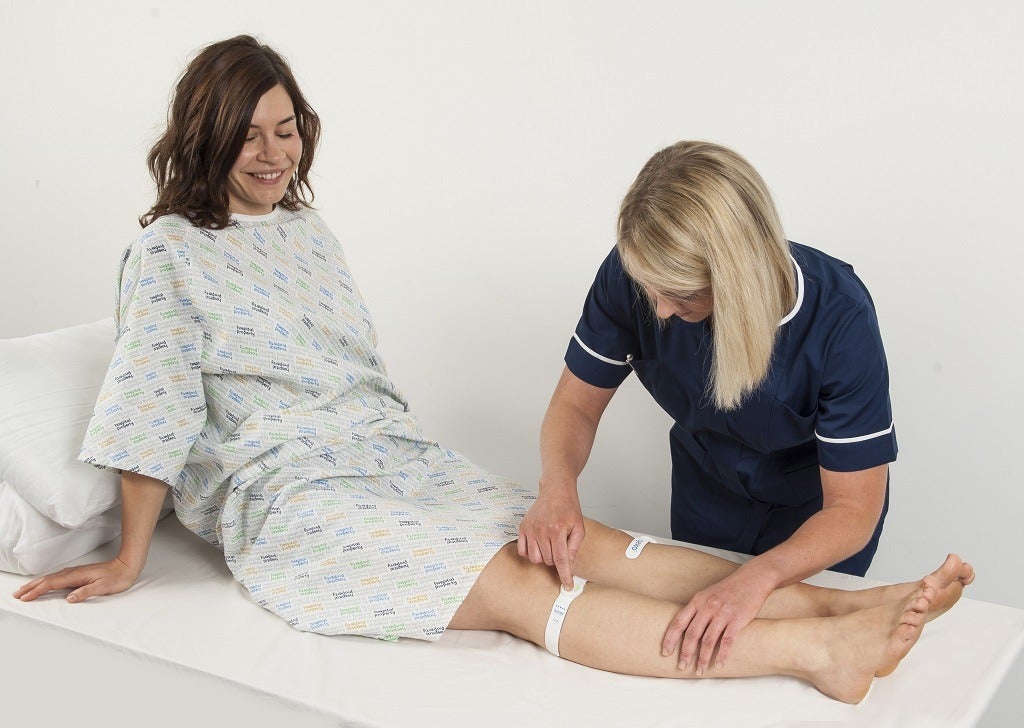
The Geko device uses electrostimulation to increase blood flow and decrease the risk of VTE (Credit: Sky Medical Technology)
The lifecycle of a medical device is rarely as straightforward as designing it, creating it and bringing it to market. Along the way, experience with the device in a clinical setting and adjustments to the manufacturing process tend to result in changes geared towards improving outcomes for the end user. That’s exactly what happened with the geko DVT prevention device sold by Sky Medical Technology, and the company’s head of product research and development Matthew Watts tells Peter Littlejohns how geko has undergone several changes since its invention.
The geko first landed in the hands of hospital staff in January 2015, fuelled by Sky Medical Technology’s ambition to serve the unmet need of patients that are unable, for practical or medical reasons, to use the hand-bag sized pumps and plastic tubes of IPC (intermittent pneumatic compression) machines.
The company’s technology uses electrostimulation to increase blood flow and venous return, preventing the blood from pooling in the legs and putting patients at risk of venous thromboembolism (blood clots in the veins), the most common type of which is deep vein thrombosis (DVT).
Some key benefits of this approach are that pulses can be delivered from a small form-factor device, meaning patients don’t have to take it off to walk around and the single-use nature of geko rules out any cross contamination – a significant benefit given the current pandemic.
But according to Matthew Watts, head of product research and development at Sky Medical Technology, the 2015 device wasn’t the first iteration, in fact a version of it was undergoing beta testing nearly five years prior, in 2011.
“The first device had to be tested in-house, we couldn’t put it on patients before we’d proven clinical safety and efficiency,” he says.
“This T1 device worked very well for certain patients and users. Firefly, which is our sport-focused device, until very recently, was still on that design.”

The US-focused Firefly device works on the same basis of increasing blood flow, but the goal is to aid athletes in their recovery by speeding up the delivery of proteins and minerals carried by the blood to heal fatigued muscles.
The second iteration, branded T2, enhanced the battery life, user interface and comfort of the strap on the device, but Watts says the main upgrade was made to overcome a limitation associated with the T1 in clinical settings.
“The core technology worked very well on people with quite skinny legs, but it struggled to stimulate people with particularly swollen legs,” he says.
“The device had to go through a redesign fairly early on because it just wasn’t stimulating enough people.”
On top of overcoming these limitations, Sky Medical Technology hired Watts, an electronics engineer by training, in 2017 to add additional features to the geko and adapt the manufacturing process to keep up with demand for the product.
DVT prevention with geko device in patients with swollen legs
The theory behind the geko device is that stimulating the peroneal nerve – a branch of the sciatic nerve which controls movement and sensation to the lower leg – will cause the calf muscle to contract and stimulate blood flow, which is key to the prevention of DVT.
The theory itself was the progeny of Professor Arthur Tucker from St Bartholomew’s Hospital and Dr Duncan Bain, with the duo releasing a paper supporting the efficacy of the device, in 2013.
“This was breakthrough technology,” says Watts.
“They went to a product design company, who we still work with to this day, and turned this idea that started off as a box and horrible looking wires on a bench into a sleek, wearable medical device.”
The engineer behind this initial consumer-friendly design change is still contracted by Sky Medical Technology.
“He designed the original circuitry, which allows us to stimulate the nerve with very few electronic components, and in a very power efficient way, which is the tricky thing,” says Watts.
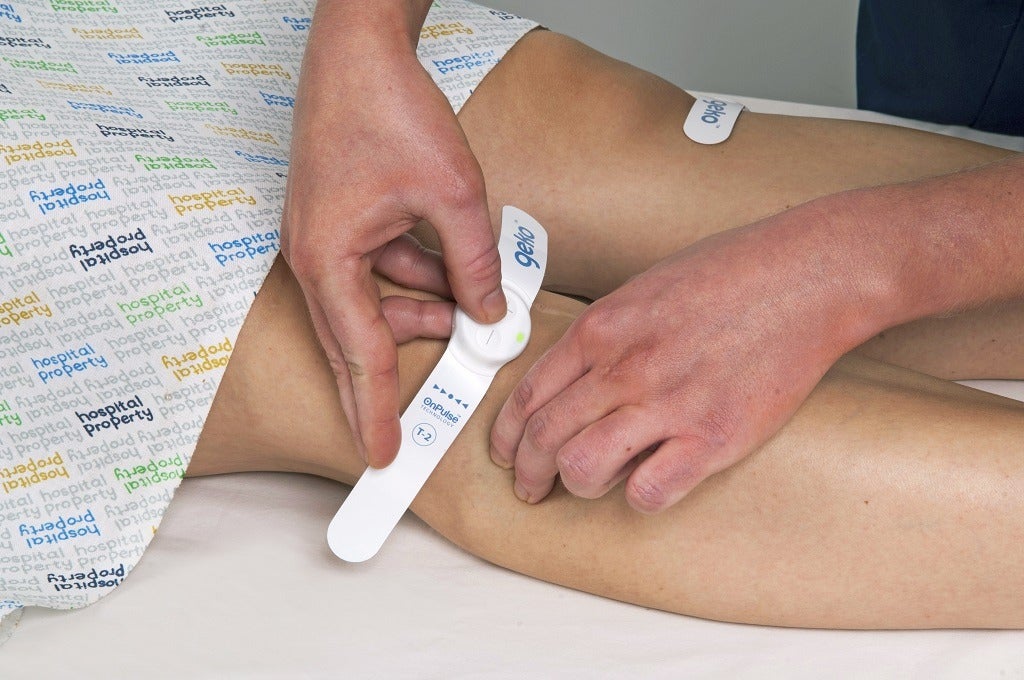
“That original circuit design is pretty much the same now.”
The key change made to the geko to overcome the issue of patients with swollen legs, a symptom of blood pooling in the legs, remains proprietary knowledge, but in essence, Watt’s says it added more of a “kick”, meaning a stronger electrical pulse was given by the device to make sure it hit the peroneal nerve in swollen legs.
It’s not just swelling this design change helped overcome though, as the extra kick from the T2 device also meant increased performance in people that were overweight, as well as for people whose peroneal nerve is located further from where the device sits on the leg.
Combining learnings from T1 and T2 to create T3
The issue at this point, says Watts, was that Sky Medical Technology had two geko devices with usage for different people, rather than one with more programmability, which is where he came in.
“We had two devices, the low stimulation one and the high stimulation one, and if someone with skinny legs puts the high stimulation one on, it’s not comfortable.
“T3, which was released about two and a half years ago, was basically a combination of all of that learning.
“We can now stimulate the hardest to stimulate people, but also those that are easily stimulated, as comfortably as possible.”
The obvious business benefit of making one multifunctional device, rather than two items geared at specific patients, is that less overall resources are needed and costs can be decreased.
But on top of this, Watts has spearheaded the addition of traceability features to the geko, giving Sky Medical Technology the ability to pull up an internal log indicating how each device has been used, which he says is useful for ensuring consistency during clinical trials and to investigate patient complaints.
“We also built in a serial number to each device, so we can trace the entire manufacturing cycle and pull up the complete test log,” he adds.
Currently, the UK’s National Institute for Health and Care Excellence (NICE) recommends the geko be used in patients with a high risk of venous thromboembolism, and for those for whom other mechanical and pharmacological methods of prophylaxis are impractical or contraindicated.
But Sky Medical Technology is continuing to conduct research to prove the geko has efficacy beyond VTE, and is currently running studies that Watts expects will be concluded inside of this year, with applications that include aiding in healing wounds and decreasing blood-clot risk in Covid-19 patients.
Using geko devices comes in at a higher cost over time than using an IPC machine because of the latter’s inherent reusability, but Watts says Sky Medical Technology is not trying to replace IPC machines, just to become the first port of call for those who can’t use them.
In the UK, the geko is used for DVT prevention in a handful of NHS hospitals for this purpose, and the company continues to grow its footprint internationally also.
