It’s no secret that deciding on a female contraceptive pill or device can involve a cost-benefit analysis of avoiding pregnancy and opening the door to several manageable yet unpleasant side effects.
For years, women have balked at the prospect of gaining weight from hormone-based contraceptives, and although there’s currently insufficient evidence to validate the criticism, established side effects can include headaches, nausea, breast tenderness, mood swings and increased blood pressure.
Despite this, a 2019 study by The Guardian found nine in ten women in the UK receiving a prescribed contraceptive were given a form of this particular medication, commonly referred to as “the pill”.
Reasons proposed by experts included women not being made aware of the various devices that can prevent them becoming pregnant, and that doctors require specialised training and more time with patients to fit them – something that’s difficult to achieve in the average 9-minute consultation window.
But while these IUDs (intrauterine device) are a popular alternative, there are several other more user-friendly innovations in female contraception – here we look at four of them.
Four companies that offer a female contraceptive device:
1. TherapeuticsMD – Annovera

While some women balk at the use of hormone-releasing contraceptives due the side effects, others are merely inconvenienced by the daily ritual involved in taking the pill.
It’s for this market that non-profit Population Council developed Annovera – a hormone-releasing vaginal ring that lasts for one year.
The female contraceptive device is inserted into the vagina, a process easily carried out by the wearer without a medical professional at hand, and is worn for three weeks and then removed, washed and stored hygienically for one, at which time the user will get their period.
When worn, the device continually releases low doses of ethinyl estradiol (0.013 mg/day) and a new kind of progestin called segesterone acetate (0.15 mg/day) into the vagina, which then enter the bloodstream and prevent pregnancy with a 97% success rate.
The device – which is licensed for commercial sale by TherapeuticsMD – also has a companion app to help women track the days they’re supposed to wear and remove it.
2. Natural Cycles – Fertility-tracking app
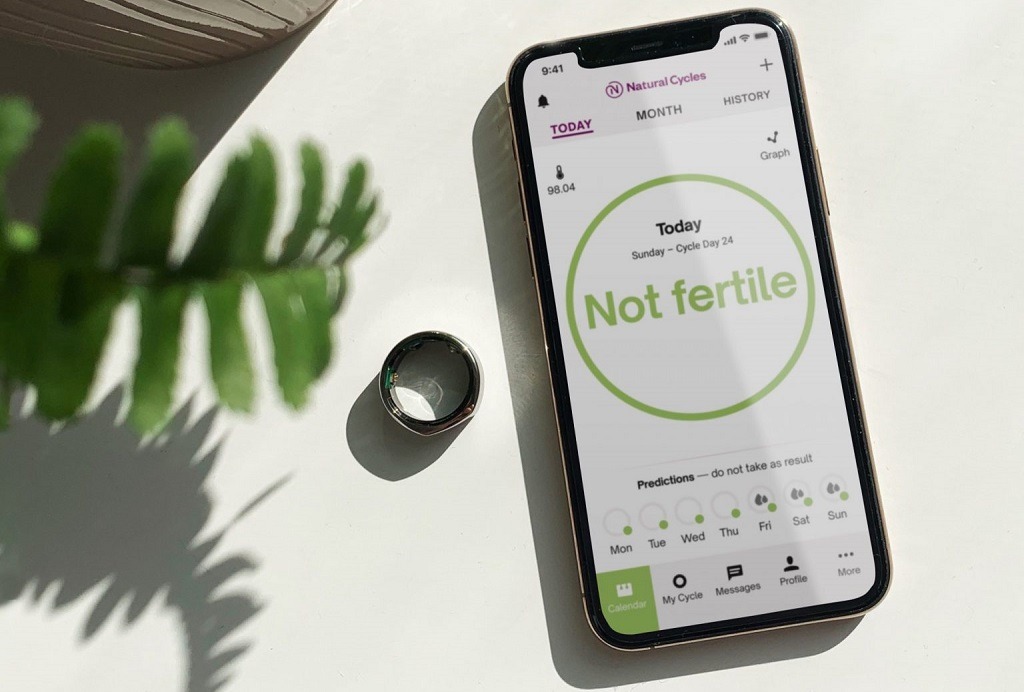
During the menstrual cycle, women’s temperatures rise after ovulation and then fall slightly when their next period comes, due to a change in their body’s hormone levels.
Natural Cycles uses an algorithm that analyses the temperature of customers as well as the period data they input to find their unique ovulation pattern, and they can also give the algorithm a boost by taking optional tests for the luteinizing hormone commonly used as an ovulation predictor.
The algorithm then takes into account other factors like sperm survival, temperature fluctuations, and cycle irregularities to identify ovulation and calculate red or green days – those in which customers are infertile or fertile, respectively.
Users currently have to take their temperature daily for best results, using a basal thermometer device and entering the figure into the app where it’s analysed and a red or green signal is given, but Natural Cycles is currently working to integrate the use of wearable tech to avoid this step.
The company claims its subscription-based app has 93% effectiveness as a method of contraception, based on clinical studies, but customers must use other methods of contraception on red days.
3. Medintim – Caya

For those women who want the benefits of contraception without the potential side effects of hormone-based products, diaphragms have been a long-standing option, albeit one that’s much less effective than the more popular methods.
Medintim is the commercial partner of global nonprofit Path, which developed Caya to improve on the traditional diaphragm design and create something that gives another contraceptive choice to women in low-income countries – although it is available in developed nations also.
The diaphragm is coated in spermicide gel and inserted into the vagina before intercourse, where it is left for 6 hours afterwards.
It can be reused for up to 2 years and was shown to have between 82% and 86% effectiveness in clinical studies.
4. Bayer AG – Ovaprene

For women that like the sound of Annovera’s ease-of-use but aren’t willing to use a hormone-based female contraceptive device, Daré Bioscience is in the process of gaining regulatory approval to bring Ovaprene to market.
Licensed for commercial sale upon approval by German medical device and pharmaceutical giant Bayer, Ovaprene is a ring just like Annovera, but instead of hormones it releases a locally-acting agent called ferrous gluconate and lasts only a month.
Ferrous gluconate impedes sperm motility almost completely, and when coupled with a unique knitted polymer barrier that physically blocks the way to the cervical canal, it prevents sperm from getting near enough to fertilise an egg.
In a 2019 post-coital study (PCT) – also known as a cervical mucous penetration test – Ovaprene was found to have blocked all sperm in the 38 female participants, but the device is currently being studied in a larger and more robust PCT study before conclusions can be drawn about its effectiveness.
The best prediction available from the company right now is that currently marketed contraceptives with similar results in their PCT clinical studies subsequently demonstrated 6-month typical use effectiveness of 86-91% in clinical trials.






