Celltrion plans to launch two rapid COVID-19 testing kits, SAMPINUTETM COVID-19 Antigen MIA for detection of SARS-CoV-2 antigen, and DiaTrust COVID-19 IgG/IgM Rapid Test for detection of SARS-CoV-2 IgG/IgM antibody in the US.
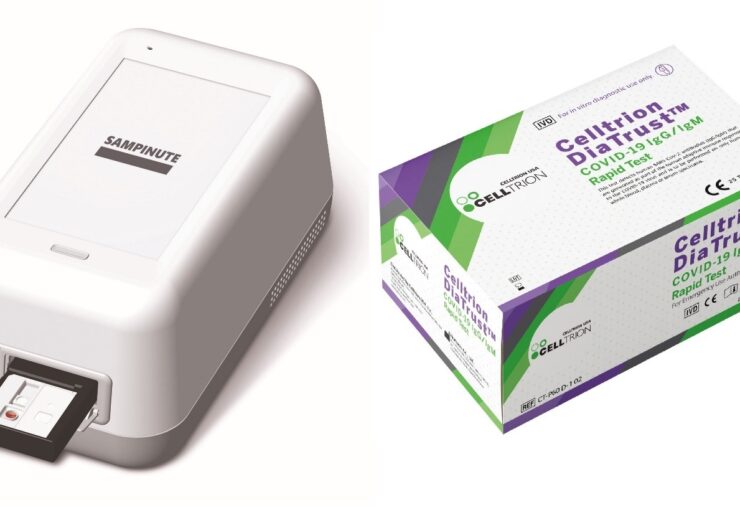
SAMPINUTE COVID-19 Antigen MIA (left) and DiaTrust COVID-19 IgG/IgM Rapid Test. (Credit: Celltrion, Inc/ Business Wire.)
Celltrion Group has announced the launch of two rapid kits for SARS-CoV-2 in the US by the third week of August.
SAMPINUTE COVID-19 Antigen MIA is an electrochemical immunoassay test for detection of SARS-CoV-2 antigen from nasopharyngeal swab samples, composed of one time use test cartridges and a portable analyzer developed in collaboration with BBB1.
DiaTrust COVID-19 IgG/IgM Rapid Test is a one-step in-vitro diagnostic test based on immunochromatographic assay designed for the rapid detection of antibodies of the novel coronavirus in healthcare settings in collaboration with Humasis.
Both SAMPINUTE COVID-19 Antigen MIA (antigen test) and DiaTrust COVID-19 IgG/IgM Rapid Test (antibody test) have shown reliable performance and promising clinical trial results. SAMPINUTE COVID-19 Antigen MIA has a sensitivity of 94% and a specificity of 100%, with time to results within 10 minutes.
DiaTrust COVID-19 IgG/IgM Rapid Test also shows reliable performance with 96% positive percent agreement and 98.67% negative percent agreement for IgM, 92% positive percent agreement and 100% negative percent agreement for IgG. The turnaround time is 15 minutes.
Celltrion requested Emergency Use Authorization (EUA) for SAMPINUTE COVID-19 Antigen MIA on July 24th, and for DiaTrust COVID-19 IgG/IgM Rapid Test on July 8th. The rapid tests kits are currently under the review of the US Food and Drug Administration’s Emergency Use Authorization. Celltrion anticipates the FDA EUA approval and subsequent commercialization in the US market by mid-August.
Celltrion is also developing a potential antiviral treatment for COVID-19 with its phase I clinical study initiated on July 17th.
Celltrion plans to launch the second generation antibody and antigen tests, in collaboration with the DiaTrust COVID-19 IgG/IgM Rapid Test’s manufacturer Humasis, for which Celltrion will apply its proprietary COVID-19 antibody-antiviral technology to enhance detection sensitivity during the second half of 2020.
“Celltrion has been striving to bring the tests into the US. The need for more accessible, affordable, and most importantly, rapid diagnostic testing will grow, as the daily activities are coming back, and the economy is opening up again,” a Celltrion representative stated. “With the short turnaround time and promising performance of the sensitivity and specificity results, Celltrion is confident that both SAMPINUTE COVID-19 Antigen MIA and DiaTrust COVID-19 IgG/IgM Rapid Test will add great value to healthcare providers in screening patients and keeping communities safe.”
Source: Company Press Release
