Uro Medical will continue Protect RCT and start Guardian clinical trial to evaluate the safety and efficacy of Protect PNS in treating OAB
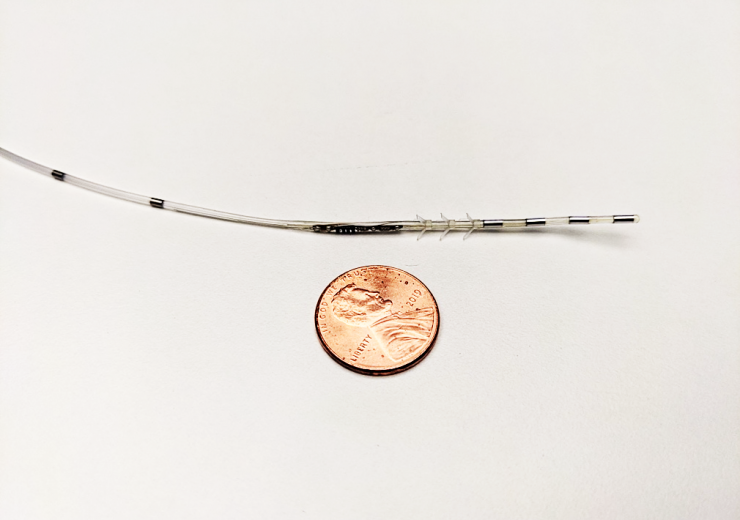
Protect PNS is a minimally invasive neurostimulator. (Credit: Uro Medical Corporation.)
US-based medical device company Uro Medical has acquired certain urology-based assets from Micron Medical for an undisclosed amount.
As part of the acquisition, Uro Medical will continue Protect randomised controlled trial (RCT), a head-to-head study that compares tibial stimulation to sacral stimulation.
Also, it will start Guardian clinical trial to evaluate the safety and efficacy of Protect PNS in treating overactive bladder (OAB), compared to traditional medical management.
Protect PNS is a wirelessly powered, minimally invasive, microtechnology neurostimulator, currently being studied for the treatment of OAB and is under regulatory review by the FDA.
Uro Medical chief commercial officer Matt Kemp said: “As a company with its foundation in neuromodulation technology, we are committed to delivering breakthrough urology products, like our Protect PNS system, that we believe will increase access, lower costs and improve outcomes for millions of patients forced to seek bladder protection solutions.
“With our Protect PNS market approval application currently under review by the U.S. Food and Drug Administration (FDA) for the treatment of patients with refractory overactive bladder (OAB), we believe we are well-positioned to deliver on the company’s urology-focused mission.”
Guardian is the second randomised clinical trial to evaluate Protect PNS device.
The study is expected to enrol around 600 participants with refractory OAB at multiple clinical sites across the US.
The company is expected to use the study results to support long term, nationwide payor reimbursement coverage for Protect PNS as a mainline therapy option.
Uro Medical engaged in the development, manufacture, and commercialisation of advanced, minimally invasive neurostimulation solutions and urological solutions.
Kemp added: “With our evolution into a fully-focused urology company complete, we are firmly dedicated to securing FDA approval of our pioneering wireless, minimally invasive, microtechnology neurostimulator, Protect PNS, for the treatment of OAB, a condition that affects over 37 million Americans, and preparing for its US commercial launch.”
In May this year, a private consortium, dubbed Pain Specialists Group, acquired Moventis PNS, the peripheral pain nerve stimulator franchise of Micron Medical.
Moventis PNS was granted the US FDA 510(k) clearance in September last year and is said to be the world’s smallest implantable and drug-free peripheral pain nerve stimulator.
