TransAeris DPS will reduce the mechanical ventilation time and frees up the vital equipment, ICU beds and clinical resources
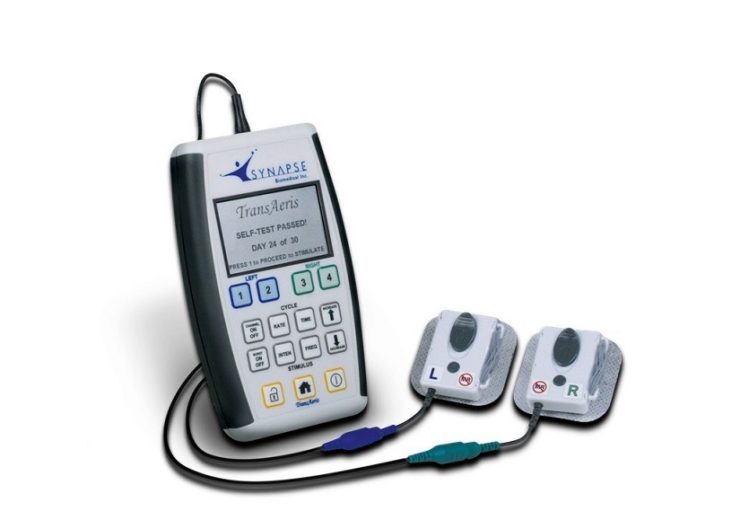
The TransAeris system. (Credit: Synapse Biomedical, Inc.)
Synapse Biomedical has received FDA Emergency Use Authorization (EUA) for its TransAeris diaphragmatic pacing stimulator (DPS), to help wean patients off of ventilators during the COVID-19 pandemic.
The technology has been recently granted CE Mark approval and is currently under clinical investigation in the US.
Synapse Biomedical president and CEO Anthony Ignagni said: “More than 2,000 patients have been successfully treated world-wide with our diaphragm, pacing technologies.
“We welcome the FDA’s leadership in providing this emergency pathway to get our latest TransAeris device into the hands of clinicians so they can help as many COVID-19 patients as possible during this pandemic.”
Synapse Biomedical has created TransAeris DPS based on its NeuRx DPS
According to the medical technology company, the rise in patients needing prolonged mechanical ventilation (PMV) during the outbreak of COVID-19 has increased the demand on hospital and ICU resources.
In addition, the PMV patients are said to be at risk of developing ventilator-induced diaphragm dysfunction (VIDD), which may further prolong their ventilation.
The TransAeris system is designed to address the issue by conditioning a patient’s diaphragm to reduce or avoid VIDD.
It has been designed based on the company’s FDA and CE Mark approved NeuRx Diaphragm Pacing Stimulation (NeuRx DPS) System, which is intended for people with Spinal Cord Injury (SCI) and has successfully reduced or eliminated the need for mechanical ventilation.
The TransAeris system is said to assist patients with prolonged mechanical ventilation on a temporary basis up to 30 days,.
It was created by simplifying the external features of the NeuRx DPS system and distilling them into a single patient, 30-day use, disposable device.
Case Western Reserve University School of Medicine chief of general surgery Raymond P Onders said: “Trauma and high-risk surgical and cardiac patients will continue to require ICU beds and ventilators that are also needed for COVID-19 patients.
“In our case, utilizing TransAeris for high risk surgical and COVID-19 patients is protecting the supply chain of ventilators, ICU beds and clinical resources by reducing the time spent on mechanical ventilators by patients at risk or experiencing prolonged mechanical ventilation.”
