The Hexanium cage is designed to reduce risk of subsidence in disc height after back surgery. It will be introduced to spine surgeons at EuroSpine 2018 and NASS 2018.
The company stated that its new product will be useful in spine fusion surgery, which is required to stop the motion of painful segment in the spine by fusing two vertebrae with a TLIF cage.
Conditions that could be treating with a spine fusion surgery include tumors, spinal stenosis, herniated discs, and degenerative disc disease.
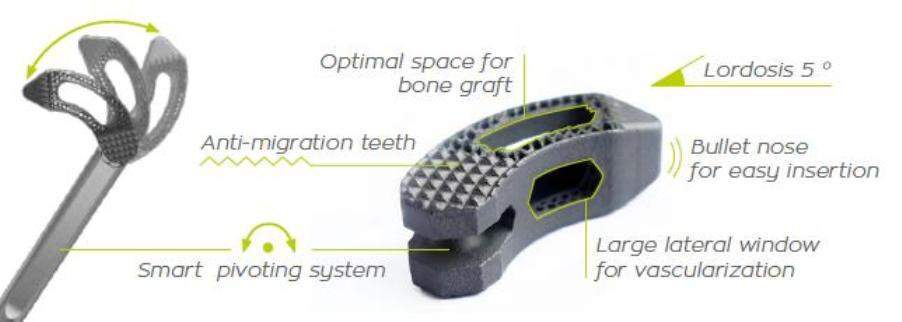
The Hexanium TLIF cage combines a roughened titanium surface designed for fast osseointegration along with a honeycomb-like structure featuring large windows to maximize bone in-growth and on-growth from endplate to endplate.
SpineVision CEO Arnaud Brisard said: “While TLIF back surgery is successful at relieving patients’ pain in about 60 to 70 percent of cases, there is room for improvement. In particular, our Hexanium TLIF cage is designed to reduce the risk of subsidence in disc height in the post-operative period.
“Hexanium represents a continuation of our substantial achievements in developing cutting-edge products for all spine pathologies. Hexanium TLIF is the first of a complete range of 3D-printed implants SpineVision will be introducing.”
According to SpineVision, spine fusion surgeries are unique among all surgeries, as there are only two types of surgeries where the goal is to have two bones while encouraging them to grow back together.
The bones will be held apart and fused together are the two vertebrae at the affected level. A cage is placed in the interbody space and packed with bone graft to help stimulate bone growth. The procedure will restore height of the spine and will stabilize the vertebrae as they fuse together.
When the procedure is successful, the bone will grow around and through the cage over time, making it the only place in the body where a material is implanted and active in the reparative process.
The company further claims that the cage and the material it is made out of play an active role in the stimulating bone growth that forms the fusion. Titanium cages with a nanotechnology surface favor factors that are associated with an anti-inflammatory response and the formation of bone.





