SIS Medical stated that its products will be sold in India in partnership with stents manufacturer Translumina Therapeutics.
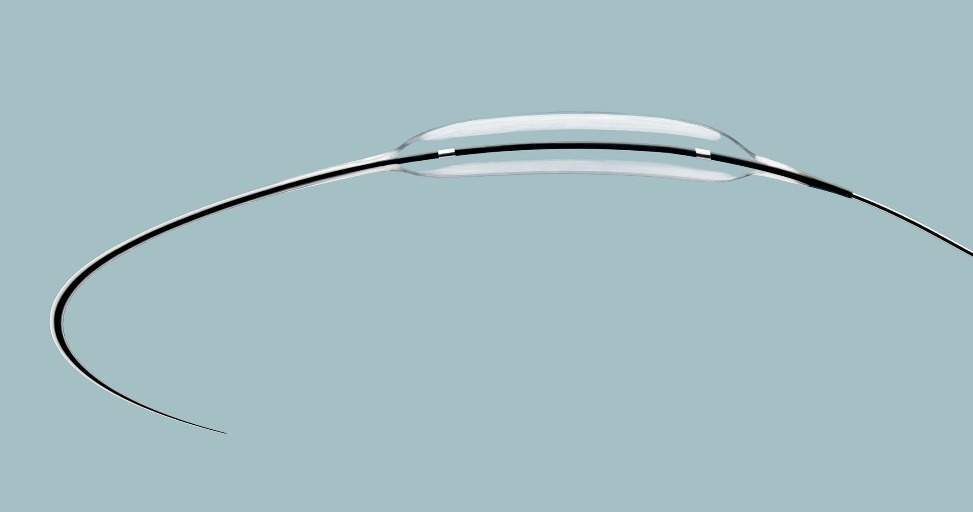
The high-pressure balloon catheter OPN NC – Super High Pressure PTCA Balloon is used in cardiology to dilate narrowed heart vessels.
It has a double balloon structure, which features high wall tension and low diameter growth.
The OPN NC is approved for a pressure of up to 35 bar and aimed at patients with calcified or severely calcified vessels.
It is claimed that the use of the high-pressure balloon catheter can lead to full restoration of the blood flow in the diseased vessels and provides the heart muscle with sufficient oxygen. Patients with coronary heart disease can improve their physical performance.
The balloon catheter is advanced to the narrowed section of the blood vessels using a guide wire and catheter and is then inflated with pressure of up to 35 bar. Conventional bypass surgery has been reduced by 80% by use of this method.
SIS Medical managing director and founder Willi Zwahlen said: “We see considerable potential for our high-pressure balloon catheters in India, one of the world’s largest markets for medical technology. Here, many people suffer from calcification of their blood vessels due to a high level of diabetes 2 and correspondingly poor nutritional habits.
“In addition, access to high-quality medicine is becoming easier and easier, and the quality of hospitals is already high and rising continuously. Our high-pressure balloon catheter can improve the lives of many people with cardiological diseases.”
The company claims that its products have been known in the Indian market for years through international congresses or their use by European doctors.
According to Indian physicians, the products will expand the therapy options for treating patients and partially replace expensive, less efficient devices and instruments.
The NIC Nano hydro balloon catheter with particularly small diameter is currently in the approval process for the Indian market. SIS Medical expects to be able to sell this balloon catheter there from April 2019.
The NIC Nano hydro only recently secured the CE marking, which allows the commercialization of the products within the European Union.




