The Sentinel continuous urine output monitor measures urine flow rate and volume in real time
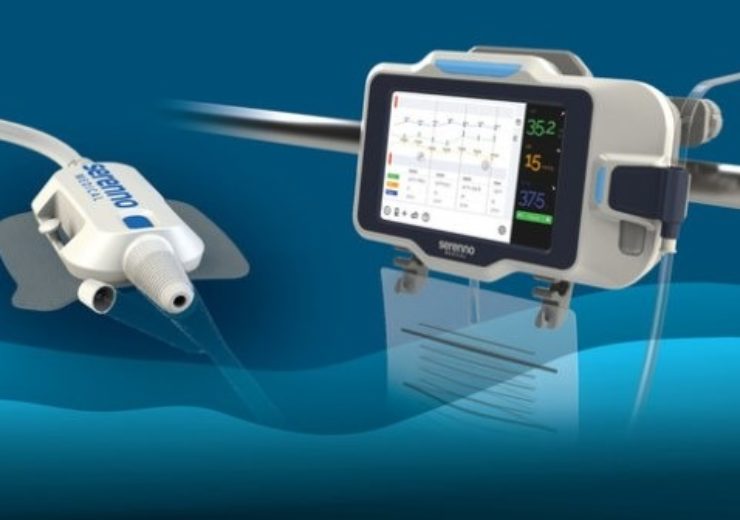
Sentinel bedside urine output monitor for early detection and prevention of acute kidney injury (Credit: Serenno)
Medical devices maker Serenno Medical has introduced the Sentinel device for continuous automatic monitoring and detection of kidney damage in hospitalised patients.
The Sentinel device is a novel continuous urine output monitor, which measures urine flow rate and volume in real-time.
The assessment of continuous kidney function enables the early detection of acute kidney injury (AKI), a common condition in hospitalised patients that may result in mortality during and after hospitalisation.
At present, the best method for monitoring kidney function is the accurate measurement of urine output (UO), which is monitored intermittently and manually by ICU nurses. The method, however, poses difficulties in detecting acute changes in urine flow with UO.
Sentinel device helps in early detection of kidney injury
Sentinel serves as a simple and cost-effective solution for the accurate and continuous measurement of urine flow rate in real-time.
The system’s ability to early detecting kidney injury will help prevent further damage to the patients.
Sentinel system helps to automatically and precisely detect small changes in kidney function, helping to reduce the burden on the nursing staff and deliver valid data.
The new device works in line with existing hospital equipment and needs a simple non-invasive installation.
Sentinel, which is suitable for the complicated intensive care unit (ICU) and operating room environments, is designed for efficient working in any patient condition or hospital environment.
Recently, Serenno Medical completed its first multicentre clinical study with the Sentinel system.
The study demonstrated the system’s reliability and accuracy in a range of environments and patient conditions, including low and high urine flow rates, regardless of patient position, during surgical procedures,
The clinical trial was carried out at three ICU units in Sheba and Rabin Medical centres in Israel. It included over 40 patients and more than1,300 hours of monitoring.
Serenno Medical chairman Dr Shimon Eckhouse said: “Serenno has developed a unique product that will bring tremendous value to patients hospitalized in ICU units along with a simple digital solution that will bring great economic value to hospitals.
“Serenno’s successful clinical trial is a great testament to the quality and performance of the Company’s product.”
In October 2019, Fresenius Medical Care North America secured breakthrough device designation from the US Food and Drug Administration (FDA) for its new haemodialysis system, which is under development.
