The remote monitoring market - which has been disrupted by Covid-19 - is set to grow by 20% between 2019 and 2025, according to analytics firm GlobalData
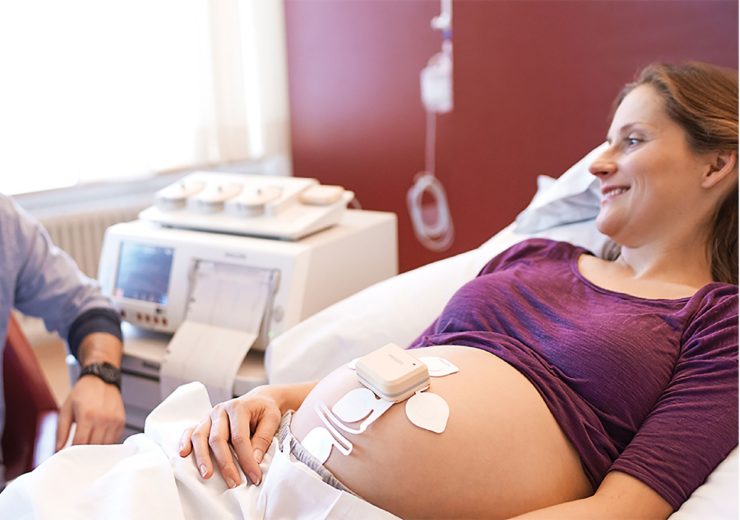
Philips launched its Avalon CL device for monitoring expectant mothers earlier this month (Credit: Philips)
Philips launching its new pregnancy monitoring device highlights the growing relevance of remote patient monitoring due to Covid-19, says an analyst.
The company’s Avalon CL obstetrics solution allows high-risk expecting mothers to be monitored outside of the hospital or clinic setting.
Remote monitoring technologies such as this are becoming increasingly sought after as healthcare professionals attempt to minimise possible patient exposure to the virus.
Dominic Tong, medical device analyst at analytics firm GlobalData, said: “The lockdown measures in place across the globe have created the perfect scenario for rapid adoption of remote monitoring technologies.
“Philip’s new solution is part of a growing remote patient monitoring market – one which GlobalData expects to grow by 20% from 2019 to a size of $645m by 2025.
“The introduction of this product, along with Philips’ other recent offerings in the remote patient monitoring space, such as a wearable biosensor for hospitalised Covid-19 patients, puts Philips in a strong position to be a market leader.”
Remote monitoring tech during Covid-19 crisis
Dutch healthtech giant Philips launched its Avalon CL fetal and maternal pod and patch obstetrics solution, which includes the firm’s perinatal analytics, visualisation software and an ultra-portable battery-operated fetal monitor, earlier this month.
It allows for continuous, non-invasive monitoring of maternal heart rate, fetal heart rate, and uterine activity with a single-use, 48-hour, disposable electrode patch, which is placed on the mother’s abdomen, and doesn’t need to be frequently repositioned like traditional elastic belts or sensors.
Philips said it hopes to reduce unnecessary physical interactions between clinicians and patients with the device – adding that this is of “particular importance” during the ongoing Covid-19 pandemic.
In May, Philips also launched its BX100 device – a wireless, wearable biosensor to help detect deterioration in hospitalised coronavirus patients.

By measuring a number of their vital signs, the wearable, disposable patch is designed to remotely monitor patients in isolation rooms, meaning clinicians do not have to come into contact with them to assess their condition.
The Philips Biosensor BX100 has received 510(k) clearance from the US Food and Drug Administration (FDA), and a CE mark for use across Europe – with the first devices being installed at the OLVG Hospital in the Netherlands.
Tong said: “The remote patient monitoring market has grown as the ongoing Covid-19 pandemic has forced healthcare professionals to seek out alternative methods of treatment, and monitoring, away from hospitals or clinics whenever possible, as they attempt to minimise potential patient exposure to the virus.
“Incumbents like Philips or Medtronic, and disruptors including Google, are looking to capitalise on this opportunity by introducing new hardware and software remote monitoring solutions in the hope of growing their market share.”
