The designation allows physician to gain expedited access to the device for patients with extremely long and complex SFA lesions
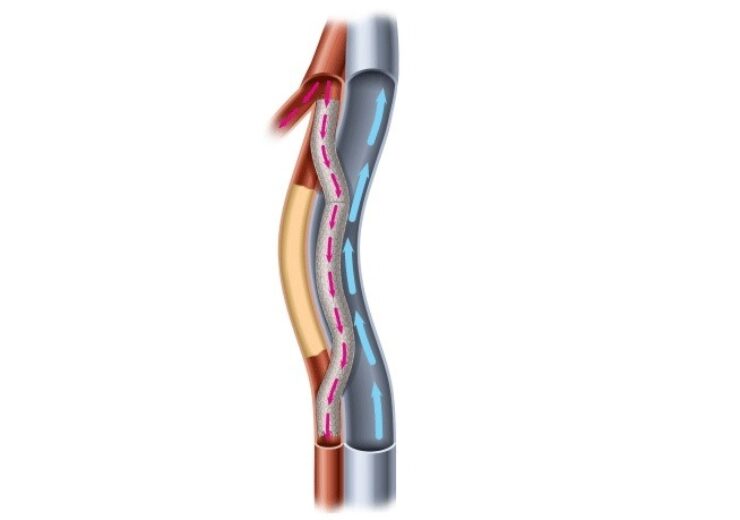
An illustration of a completed Detour Procedure within the Superficial Femoral artery and the Femoral Vein (Credit: Business Wire)
Medical device company PQ Bypass has secured breakthrough device designation from the US Food and Drug Administration (FDA) for its fully-percutaneous femoral-popliteal bypass device called Detour System.
The Detour System is claimed to be the world’s first fully-percutaneous femoral-popliteal bypass device designed for the treatment of extremely long and complex blockages in the superficial femoral artery (SFA).
Detour System will help treat patients who are unable to conduct their daily living activities
PQ Bypass has developed the Detour System to treat patients who are not in a position to conduct their daily living activities, as well as unable to enjoy their liberties of free mobility due to advanced symptomology, severe lesion morphology, and multiple co-morbidities.
According to the company, revascularisation becomes imperative to mitigate the ongoing deterioration and to prevent amputation, once PAD advances to debilitating claudication or tissue loss.
If not treated, these patients may face further functional deterioration, major adverse limb events, and mortality.
Bypass chairman and CEO Rich Ferrari said: “We are nearing enrollment completion in our DETOUR2 and TORUS2 IDE studies, and had new positive data on our technology published in the Journal of Vascular Surgery, making 2020 a momentous year for the company.
“This is an exciting time for all of us at PQ, with this important designation a credit to such a talented group. We are honored and proud to be part of this important journey to develop a first-line therapy in the treatment of advanced PAD.”
In October 2019, PQ Bypass secured an approval from the FDA for its investigational device exemption (IDE) trial of TORUS stent graft.
TORUS stent graft is an advanced stent-graft platform designed for the treatment of peripheral artery disease (PAD) in the superficial femoral artery (SFA).
