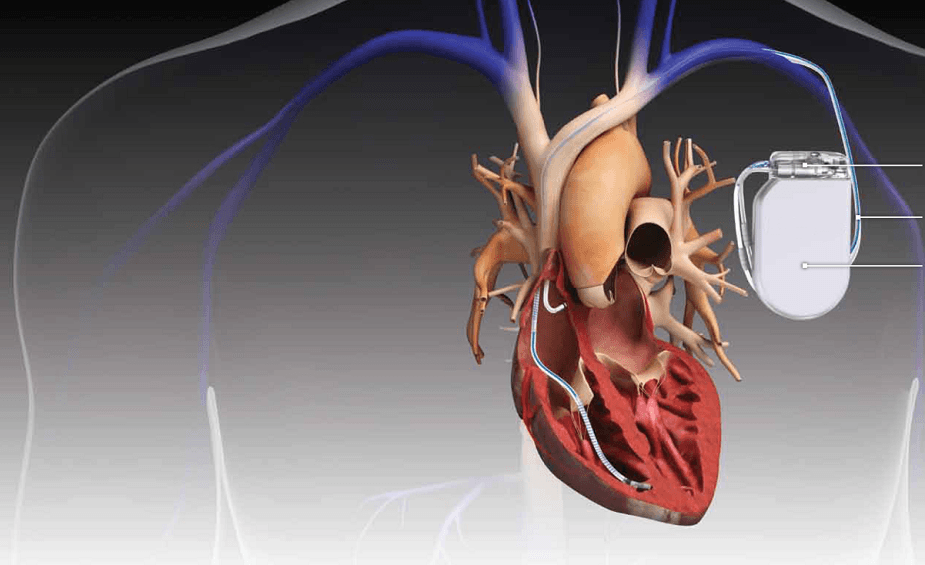
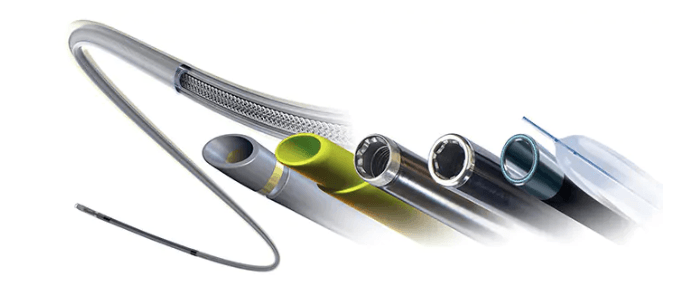
Philips is planning to release the new TightRail Guardian laser sheath, which helps clinicians make a significant positive impact for patients when removing and replacing leads and devices during cardiac implant procedures.
The medtech’s lead management device has been submitted for FDA clearance, ahead of the Heart Rhythm Society’s annual scientific sessions, currently taking place in San Francisco, where it is being showcased by Philips.
Expansion in the use of cardiac implantable electronic devices (CIED), including pacemakers and implantable cardioverter defibrillators, has resulted in an estimated 13 million cardiac implanted electronic device leads worldwide.
In the US alone, CIEDs have evolved to be a prevalent treatment option for chronic cardiac patients, with more than 300,000 individuals receiving new CIED implants every year, according to Journal of American College of Cardiology.
Chris Duffie, Business Segment Leader for Lead Management at Philips, said: “As soon as a device is implanted, the patient’s anatomy starts to adapt.
“Over time, attachments form between the leads and the vessel walls, making their removal difficult and particular to each individual.
“Starting with the introduction of our laser system in 1997, we have led the way, continuously innovating in lead management and providing physicians with tools that are designed with safety in mind.”
What is Philips’ TightRail Guardian laser sheath?
With advancing technology and increasing insertion of CIEDs, the demand for lead extraction is growing as higher numbers of patients receive more complex devices with multiple leads per patient.
Most leads travel through a vein to enter the right side of the heart by latching onto its interior, and then screwed directly into the muscle of the heart wall.
Lead extraction has emerged as an important procedure for lead management, particularly for patients with CIED-related infections.
There are many reasons why both leads and devices may need to be removed and replaced during a patient’s lifetime, and these procedures require a high level of expertise, with physicians requiring a wide range of tools to provide a tailored solution for each patient’s specific needs.
Philips’ laser sheaths incorporate optical fibres arranged in a circle, with the energy emitted from the tip removing tissue holding the lead and freeing it in a controlled method.
It claims to offer “the only laser sheath indicated for use in lead extraction procedures”.
The lead management device also includes a “shielded mode” designed to prevent the dilation mechanism from contacting the vessel wall.
Mr Duffie said: “Our portfolio already includes a wide range of extraction solutions – laser, mechanical and manual sheaths that travel over the lead to free it from vessel tissue and easily remove it from the body.
“The TightRail Guardian was designed based on clinician feedback to address some of the challenges with lead extraction.
“We firmly believe in managing every lead, safely, predictably and responsibly, and with our broad portfolio of tools, we’re supporting clinicians to provide the best care to their patients.”






