The DEFINE GPS study will assess patient outcomes after receiving a PCI guided by Philips’ co-registration platform
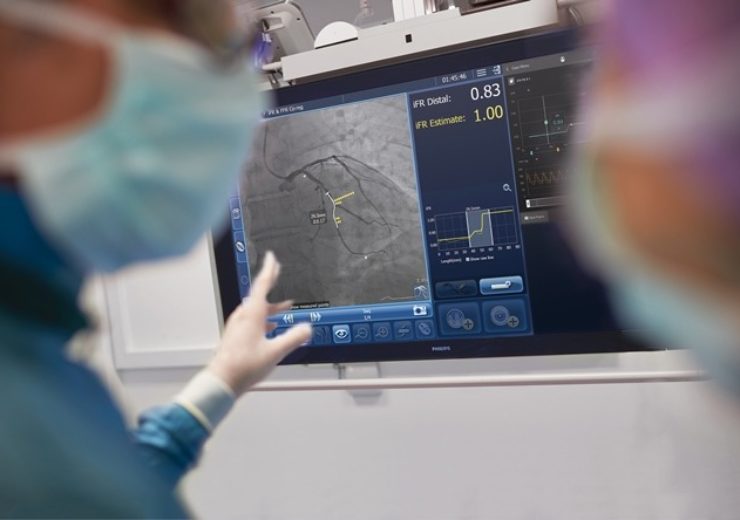
The DEFINE GPS study will assess the use of iFR in conjunction with the Philips Image-Guided Co-registration Systemfor PCI guidance (Credit: Koninklijke Philips N.V.)
Royal Philips has announced that it will begin a new randomised controlled study to evaluate patient outcomes of percutaneous coronary intervention (PCI) guided by integrated instant wave-Free Ratio (iFR) and interventional X-ray images.
The trial will evaluate patient outcomes after receiving a PCI guided by Philips’ co-registration platform, which aggregates data of an instant iFR measurement and the angiogram (interventional X-ray image compared against the current standard of care treatment guided by an angiogram alone.
The DEFINE GPS (distal evaluation of functional performance with intravascular sensors to assess the narrowing effect: guided physiologic stenting) study will assess the use of iFR in conjunction with the Philips Image-Guided Co-registration System (SyncVision) for PCI guidance and the enhancement of treatment outcomes.
Sponsored by Philips with the Cardiovascular Research Foundation, the trial will recruit the first patients in the second half of 2020.
DEFINE GPS study principal investigator Dr Allen Jeremias said: “However, when we look back at all the major, high-quality stent trials over the past 20 years we see that around 20-30% of patients continue to have recurring chest pain at one year after receiving treatment.”
The DEFINE GPS trial will evaluate the impact of iFR co-registration on both outcomes and cost-effectiveness
DEFINE GPS is a global multicentre, prospective and randomised controlled trial that will assess the impact of iFR co-registration on both outcomes and cost-effectiveness.
The target vessel failure or rehospitalisation for progressive or unstable ischemia at two years is the primary endpoint of the trial.
Philips image-guided therapy devices general manager and senior vice president Chris Landon said: “iFR continues to be adopted into clinical practice, with mounting evidence that this innovative technology contributes to improving outcomes, reducing costs [1, 2, 3] and enhancing the patient experience.
“This major study will provide a definitive answer to the question of the overall improvement resulting from the use of a functional guidance strategy on patient outcomes and cost.”
Last month, Philips announced the launch of new data-driven personalised connected care solutions for sleep, oral health and mother and child care.
