Now accessible to AllSpire's members, the MolecuLight i:X and DX wound imaging devices will assist physicians to improve the state of wound care and the end results
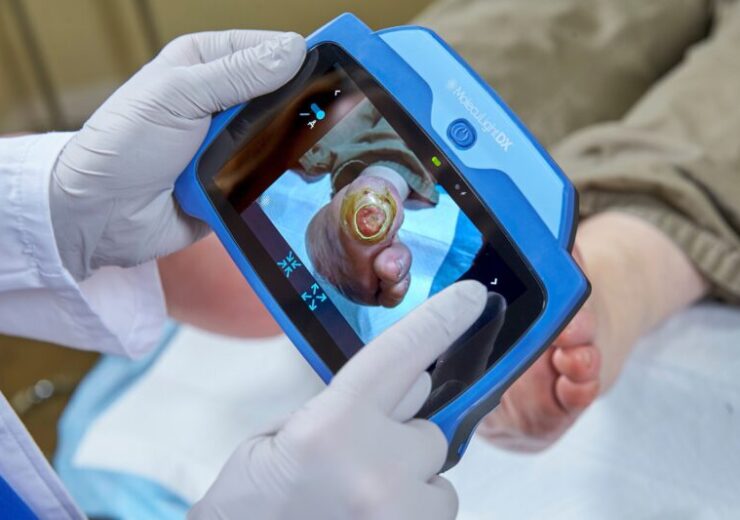
MolecuLightDX point-of-care imaging system which detects increased bacterial loads in wounds. (Credit: PR Newswire Europe Limited/MolecuLight Inc.)
Medical imaging company MolecuLight has secured a new group purchasing agreement with AllSpire Health GPO, a US-based group purchasing organisation, for its MolecuLight i:X and DX wound imaging devices.
Now accessible to AllSpire’s members, the two imaging devices will assist physicians to improve the state of wound care and eventually improve end results.
AllSpire Health GPO strategic sourcing senior director James Wallick said: “We are most impressed with the clinical utility that the MolecuLight i:X and DX devices provide to wound care professionals and are pleased to offer the MolecuLight portfolio via our Group Purchasing Agreement to our member hospitals.”
The MolecuLight imaging devices enable doctors to see the presence, location, and load of bacteria in wounds in real-time.
According to data from MolecuLight’s recent 350-patient, 14-site clinical trial, the clinical standard of care alone recognised 15% of wounds with increased bacterial load, but the inclusion of the MolecuLight device resulted in a 400% improvement in wound detection.
The imaging firm said that the i:X and DX offer vital bacterial data at the point of care that can guide clinical judgement and enable targeted wound therapy.
In a 2022 randomised controlled trial, the improvement in healing rate at 12 weeks was two times more in patients whose care was guided by MolecuLight fluorescence imaging compared to those that were not. There have been reports of improvements in the patient’s quality of life also, said MolecuLight.
The medical imaging firm said that another study reporting improved healing rates when bacterial information from MolecuLight imaging was used, also discovered much less need for antimicrobial dressings and systemic antibiotics.
Additionally, the company’s devices are said to enable precise digital wound measuring to enable consistent wound monitoring and their documentation.
MolecuLight CEO Anil Amlani said: “Through the i:X and DX, we hope to enable significant cost-savings and improvements in clinical outcomes.
“AllSpire’s extensive member base can now easily access the MolecuLight wound imaging devices and see the clinical benefits in their wound care practices.”
In June this year, the firm received an expansion to the US Food and Drug Administration (FDA) 510(k) approval for the MolecuLight i:X imaging device.
