The US health agency’s expanded approval of the MolecuLight i:X imaging device is based on a detailed retrospective statistical analysis of more than 350 patients
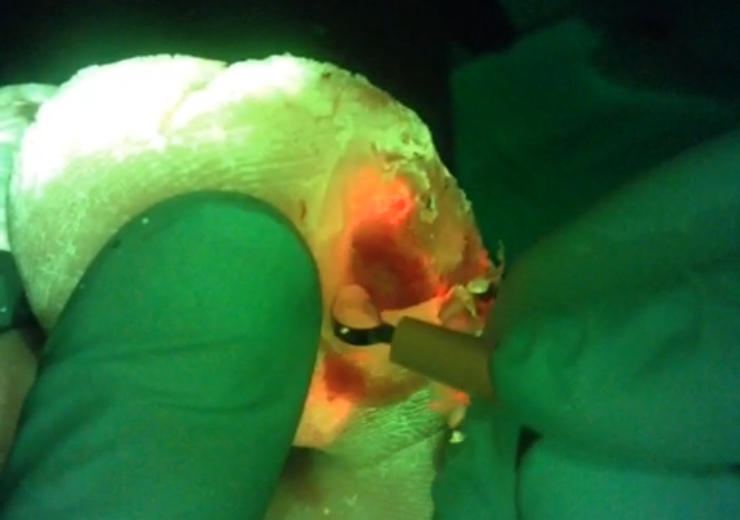
MolecuLight i:X to identify regions with elevated bacterial load. (Credit: MolecuLight Inc.)
MolecuLight has secured an expansion to the US Food and Drug Administration (FDA) 510(k) approval for the MolecuLight i:X imaging device to include the detection of regions with elevated bacterial loads in wounds.
In addition, the label expansion includes the device’s ability to identify areas of the wounds that host more bacterial species, including target pathogens of interest to the CDC.
The expanded approval is based on a detailed retrospective statistical analysis of more than 350 patients, said the medical imaging company.
MolecuLight CEO Anil Amlani said: “We are thrilled with the FDA’s new clearance for MolecuLight’ ability to determine the location of elevated bacterial loads in wounds, in addition to the ability to identify regions with more bacterial species of interest.
“Clinicians worldwide are using the MolecuLight device to visualise regions with clinically significant bacterial loads and more species of concern.
“With point-of-care information on bacterial load and its locations through the use of a MolecuLight device, clinicians can act immediately to tailor their cleaning, debridement, antimicrobial strategies and treatments accordingly.”
MolecuLight said that its imaging device will highlight the regions with high levels (>104 CFU/g) of bacterial load in red, which is visible on the patient’s diabetic foot ulcer.
The device is said to help clinicians with their decision-making, and target their wound hygiene to the areas of red fluorescence.
Also, the US FDA has confirmed MolecuLight’s ability to visualise regions with harmful bacterial species at the point of care, said the company.
MolecuLight i:X can be used to perform fluorescence-guided tissue biopsies in the highlighted regions to detect a large number of pathogens of interest to the CDC.
The CDC considers antibiotic resistance a major global public health challenge.
MolecuLight is a privately-owned medical imaging company that develops and markets its unique fluorescent imaging platform technology in multiple clinical markets.
Its suite of devices includes the MolecuLight i:X and DX fluorescence imaging systems, and point-of-care handheld imaging devices for real-time monitoring of wounds.
