The Mitra is a medical device intended for remote use by anyone to collect a small blood sample using a simple finger-stick method
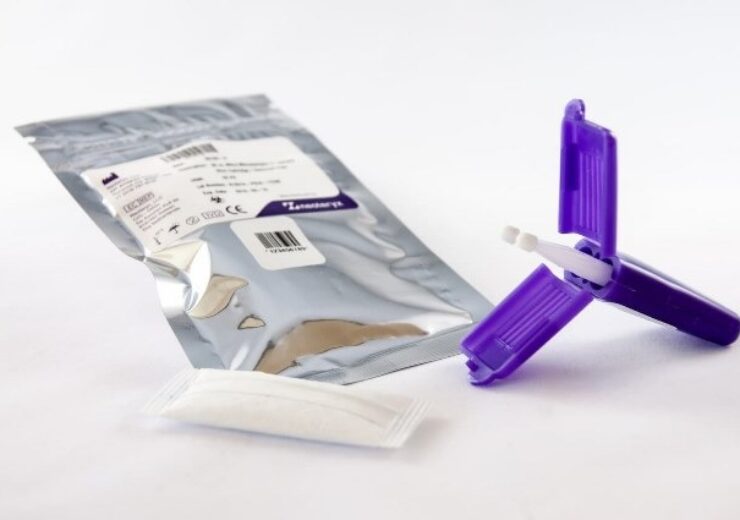
Mitra devices come in a plastic cartridge that protects specimen samples during collection, shipping and storage. (Credit: PRNewswire / Neoteryx)
The Mitra® microsampling device from US-based Neoteryx is now registered as a Class I in vitro diagnostic (IVD) medical device with the Chinese Health Authority in China. The Mitra device met all regulatory approval requirements of the National Medical Products Administration (NMPA), formerly known as the China Food and Drug Administration.
The Mitra is a medical device intended for remote use by anyone to collect a small blood sample using a simple finger-stick method. The Mitra device has an absorbent tip that is designed with volumetric absorptive microsampling technology.
The patented VAMS® tip on the Mitra device allows even untrained users to easily collect a fixed-volume microsample of blood that is precise enough for analysis in a lab. Mitra devices can be used at home or out in the field and are currently used around the world for remote research studies, clinical trials and programs, and public health surveys.
Research centers, laboratories and hospitals or clinics in China can send Mitra devices to their research volunteers or patients who wish to remain safe at home or who live in remote areas without transport. With Mitra devices available in China, researchers and healthcare professionals will be able to get blood samples without requiring people to visit a hospital or facility for an in-person blood draw. The small Mitra device makes it easy for anyone to collect a high-quality blood sample for validated lab testing. The data gathered from Mitra microsamples can be used for scientific research and clinical diagnostics.
“The Chinese NMPA registration confirms that Mitra® devices from Neoteryx have met all regulatory and quality standards in China and can be imported into the country for use in both research and clinical applications,” said Loc Huynh, MS, RAC, Regulatory Affairs and Quality Director, Neoteryx.
After specimen microsamples are collected onto Mitra devices, they are stored and shipped inside a protective plastic cartridge, which is sealed in a specimen bag that fits into a large mailing envelope for sending to a lab via regular mail. Mitra microsamples are analyzed in the lab as dried blood samples. Dried blood microsamples can be used for serology studies, therapeutic drug monitoring, and managing chronic illness such as diabetes or cardiovascular disease. Remote specimen collection with Mitra devices is ideal for remote patient monitoring of pediatric or elderly patients or for virtual clinical trials and public health studies where people can participate from home.
Source: Company Press Release
