Three orthopaedic products made by Implants International have received an MHRA safety alert after being sold without valid CE certification
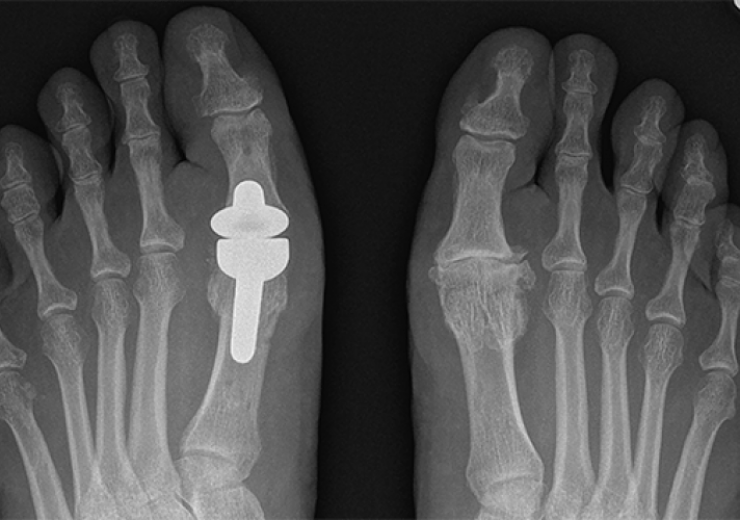
One of the devices covered by the MHRA alert is the Roto-Glide Great Toe MTP implant system - a lower-extremity orthopaedic product (Credit: Implants International)
The MHRA has released a device safety alert concerning three orthopaedic implants, made by Implants International, that have not received valid CE certification since 2019.
The regulator said it’s aware the products in question have been distributed to several hospitals in the UK – despite the fact their safety and efficacy “cannot be assured”.
After discovering that UK-based manufacturer Implants International had continued to sell them after 4 September 2019, when the relevant CE certificate was withdrawn, the MHRA sent a targeted letter to the specific organisations it knows currently have these devices.
The three devices covered by the MHRA alert include two lower-extremity orthopaedics – the Roto-Glide Great Toe MTP (metatarsophalangeal) implant system and the Footlocker midfoot & forefoot plate & screw implants – and one nailing product, the Omni-Fix IM (intramedullary) nail system.
The regulator stated these implants and the associated surgical instruments “should not be used in the UK”, and has advised organisations to return them to the manufacturer – adding that, if any have been implanted into patients, organisations should consider the need for continued clinical follow-up and monitoring for adverse reactions.
In the safety alert, the MHRA also said: “We understand that the manufacturer is currently in the process of obtaining new certification.
“Once this process has been successfully completed, the MHRA will review the prohibition status.”
MHRA recommendations for Implants International products
Due to the unknown risks associated with the three devices listed above, the MHRA provided the following recommendations to affected hospitals and healthcare institutions:
- Do not implant any of the affected devices.
- If you have any devices remaining on site, remove them from stock and return them to the manufacturer – Implants International Ltd, trading as Xtremity Solutions Ltd.
- Consider the need for continued clinical follow-up and monitoring for adverse reactions in patients who have been implanted with affected products distributed after 4 September 2019.
- Report any suspected or actual adverse incidents involving these devices through your healthcare institution’s local incident reporting system and your national incident reporting authority as appropriate.
