PulseSelect is a minimally invasive cardiac ablation system engineered with differentiated safety features to provide rapid pulmonary vein isolation (PVI) through consistent and predictable energy delivery and catheter manoeuvrability
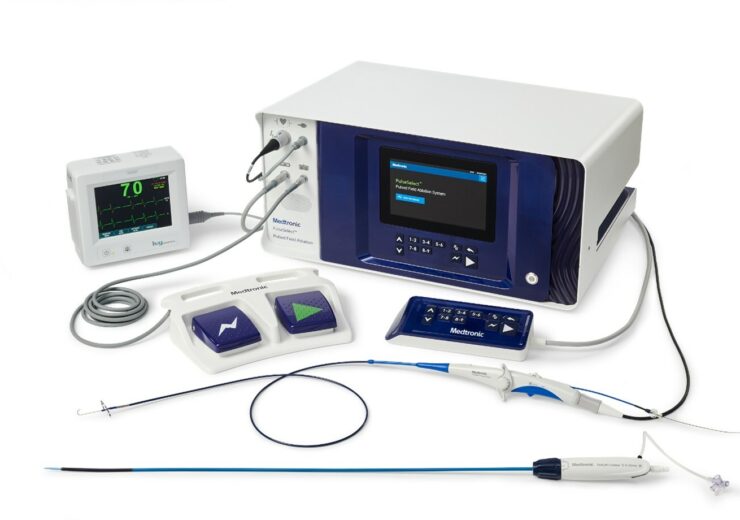
Medtronic PulseSelect PFA System. (Credit: Medtronic)
Medtronic has received the US Food and Drug Administration (FDA) approval for its PulseSelect Pulsed Field Ablation (PFA) System to treat both paroxysmal and persistent atrial fibrillation (AF).
PulseSelect is a minimally invasive cardiac ablation system that provides rapid pulmonary vein isolation (PVI) through consistent and predictable energy delivery and catheter manoeuvrability.
Medtronic said the PFA system is engineered with differentiated safety features to enable an easy transition to PFA in a clinician’s preferred workflow.
Also, PulseSelect is the first PFA technology to receive FDA approval, which follows the granting of FDA Breakthrough Device Designation and CE mark approval.
The medical device maker intends to commercialise the PFA system in early 2024.
Medtronic SVP and cardiac ablation solutions business president Rebecca Seidel said: “Launching the first FDA-approved PFA technology is not just a milestone; the PulseSelect PFA system is setting a new standard in safety for AF ablation with excellent efficacy and efficiency.
“The PulseSelect PFA system, together with the CE Marked Affera mapping and ablation system and our strong Cryo platform, enables us to provide a broad portfolio of solutions to clinicians and their patients, all developed with years of research and supported by compelling scientific evidence.”
Medtronic cardiac ablation solutions business chief medical officer Khaldoun Tarakji said: “What motivates all of us at Medtronic is the privilege of serving patients by empowering electrophysiologists globally with the safest and most effective ablation technologies that seamlessly integrate with their workflows and enable them to tailor therapy based on their patients’ needs.”
The PulseSelect PFA system is designed as a plug-and-play system and can be used with any mapping system or with just fluoroscopy.
It comes with built-in safety features, such as a phrenic nerve test pulse, and fixed spacing for the small, nine-electrode, 9Fr bidirectional catheter.
The phrenic nerve test pulse is a non-therapeutic low-voltage pulse that enables the evaluation of catheter proximity to the phrenic nerve before delivering the actual therapy.
The 9Fr bidirectional catheter produces a predictable and consistent electric field for contiguous ablation and can also be used for pacing and sensing.
It enhances manoeuvrability and access to various anatomical structures and is compatible with a 10Fr sheath, including the custom bidirectional FlexCath Contour sheath.
Medtronic validated the safety, efficacy, and efficiency of the PulseSelect PFA system in the PULSED AF clinical study.
In the study, the PFA system showed a 0.7% safety event rate and clinical success rate of 80% in both paroxysmal and persistent AF patients.
PULSED AF trial operator Amin Al-Ahmad said: “The PulseSelect PFA system ushers the EP community to a new era of safe, effective, and efficient AF ablation that overcomes many challenges in our current practice.
“In my clinical experience with the catheter, it was designed for AF ablation procedures. The learning curve in using the catheter and system is short, and the catheter enables the operator to deliver fast and controlled pulsed field energy for AF ablation.”
