The new wearable wireless patient orientation, activity, and respiration sensor is designed to help clinicians monitor patient position and respiration rate
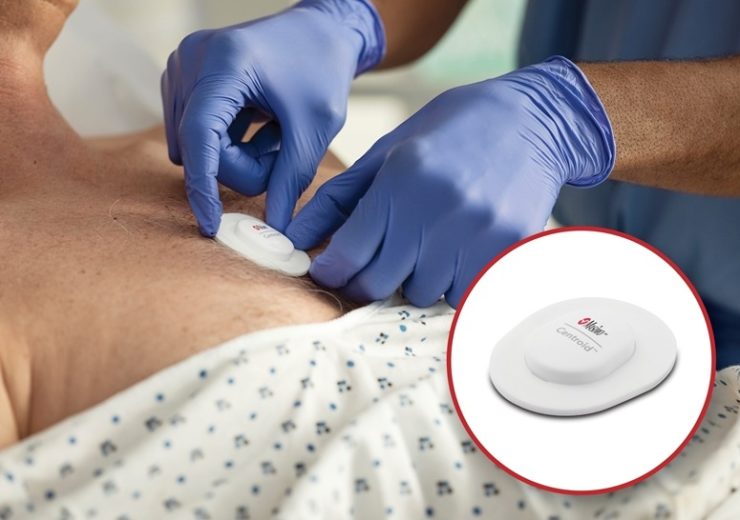
Masimo Centroid for the orientation monitoring of patients. (Credit: Business Wire.)
Masimo has received the US Food and Drug Administration (FDA) approval for its new wearable, wireless patient orientation, activity, and respiration rate sensor, dubbed Centroid.
Centroid is indicated for the orientation monitoring of patients who are vulnerable to pressure ulcers, by tracking patient movement and activity using an accelerometer and gyroscope.
The device is said to help clinicians monitor patient position to avoid preventable pressure ulcers, and can alert clinicians to sudden movements including fall-like events.
In addition, the advanced censor will facilitate detection of chest movements to continuously provide respiration rate, and assists clinicians with additional data to support the care decisions.
Masimo founder and CEO Joe Kiani said: “We are committed to using our expertise in signal processing and sensor design to develop new ways to provide the highest quality, most relevant data to clinicians in the most intuitive, useful formats, and Centroid, coupled with Root’s rich high-resolution display, is a great example of this.
“We hope that by helping to automate the process of tracking and making decisions about patient position, we can help clinicians reduce the frequency and severity of pressure ulcers, and ultimately improve patient outcomes.”
Centroid is a single-patient-use sensor designed for continuous use during daily activities
Centroid is a single-patient-use sensor that is ergonomically designed for application on the chest using flexible, lightweight material for patient comfort, and supports continuous use during daily activities.
The battery-operated sensors are designed to last four days, reducing the need for frequent replacement.
The sensor is capable of identifying patient’s position and orientation to the nearest degree, with alerts based on the duration in a static position to support the adherence to hospital patient turn protocols.
To monitor patient position and detect changes in position, the device can be paired with the company’s Root patient monitoring and connectivity platform via Bluetooth to facilitate tracking of a patient’s posture, orientation, and activity.
The data transmitted by the sensor can be displayed in different formats on Root, helping clinicians assess adherence to protocols related to tissue stress and offer customised care for specific needs of each patient.
