The deal allows Magstim to purchase EGI hardware, software and sensor nets assets
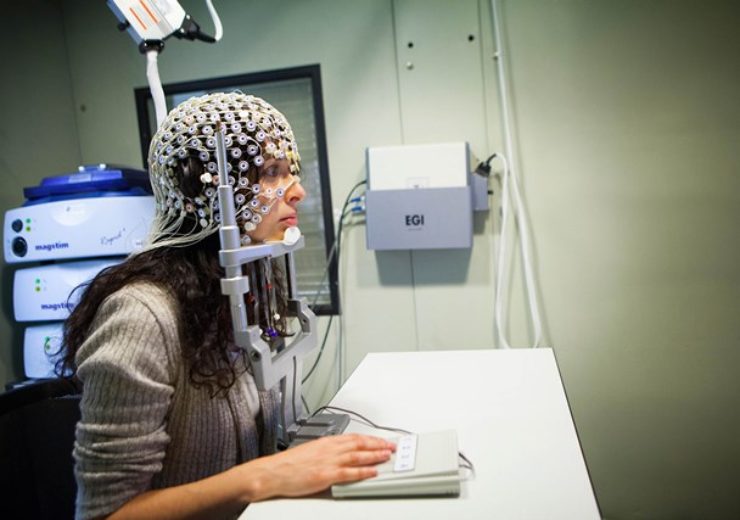
Magstim has acquired EGI’s product portfolio from Royal Philips. (Credit: Magstim)
Magstim, a provider of transcranial magnetic stimulation (TMS), has agreed to acquire Electrical Geodesics (EGI) product portfolio from Royal Philips for an undisclosed sum.
EGI is engaged in the development of non-invasive multimodal technologies for the monitoring of brain activity. It also offers transcranial electrical stimulation required for brain research.
Under the deal, Magstim will purchase EGI hardware, software and sensor nets assets, as well as immediately begins the management of global sales, service and support. Philips is said to support the transition through the end of this year.
Electroencephalography (EEG) is applied for the assessment of the electrical activity in the brain.
EGI’s advanced dense array EEG technology collects data from more electrodes than conventional EEG.
The neuroscience researchers use EGI’s Geodesic Sensor Net to generate high-resolution data
The neuroscience researchers across the globe are using EGI’s Geodesic Sensor Net (GSN) for the generation of high-resolution data.
Magstim Group CEO Lothar Krinke said: “This adds high-density EEG to our product portfolio supporting more than 1,200 research labs and clinics that focus on mental health, brain disorders and cognitive neuroscience.
“Magstim has been pioneering and advancing transcranial magnetic stimulation for 30 years. High-density EEG closes the loop for the development of a comprehensive system for non-invasive neuromodulation. Magstim and EGI share a legacy and passion for innovation in neuroscience.”
Magstim will continue to operate from EGI’s Oregon office, which is supported by Magstim’s global offices in the UK, Netherlands and Minneapolis. Magstim’s sister brands comprise of Neurosign and Technomed.
Magstim is supported by San Francisco based private equity company Telegraph Hill Partners. It is also a member of Medical Alley, the health technology innovation cluster and home to the nation’s largest private health insurer and over 1,000 healthcare companies.
In June, Royal Philips secured emergency use authorisation (EUA) from the US Food and Drug Administration (FDA) for new acute care patient monitoring solutions to be used for Covid-19 patients.
