Philips’ patient monitoring solutions are designed to deliver critical patient information for caregivers to help better treat hospitalised Covid-19 patients
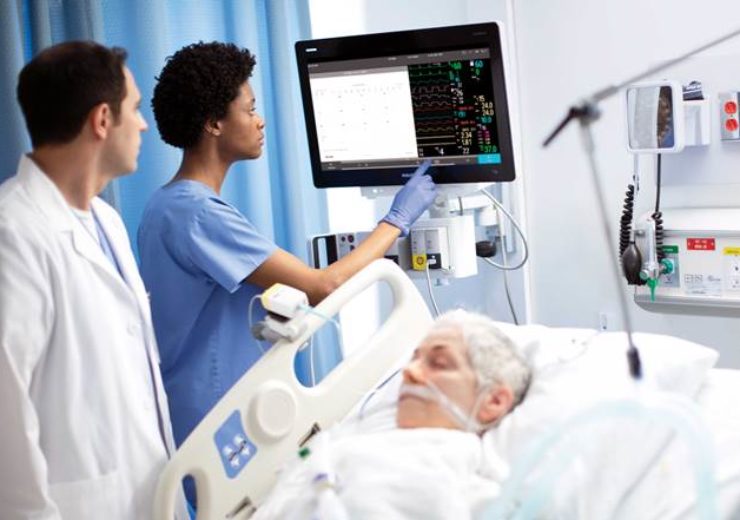
Philips IntelliVue MX850 monitor. (Credit: Koninklijke Philips N.V.)
Royal Philips has secured emergency use authorisation (EUA) from the US Food and Drug Administration (FDA) for new acute care patient monitoring solutions to be used for Covid-19 patients.
The EUA status enables to use Philips’ IntelliVue Patient Monitors MX750/MX850 and its IntelliVue Active Displays AD75/AD85 in the US during the Covid-19 health emergency.
Philips’ patient monitoring solutions are said to support infection-control protocols and remotely offer critical patient information for caregivers to help better treat hospitalised Covid-19 patients.
Last year, the company secured CE mark approval for IntelliVue Patient Monitors MX750/MX850 and IntelliVue Active Displays AD75/AD8 for use in hospitals across Europe.
The EUA enables to start supplying the new remote patient monitoring solution to hospitals in the US. It is also planning to submit a 510(k) to FDA for this acute care solution during this year.
Philips’ new patient monitoring solutions deliver advanced clinical decision support capabilities
The IntelliVue Patient Monitors MX750/MX850 and IntelliVue Active Displays AD75/AD85 deliver advanced functionality and clinical decision support capabilities such as Philips’ IntelliVue Horizon Trends information view, which display deviations in vital signs to contextualise a patient’s condition.
Philips’ Alarm Advisor and Alarm Reporting features enable to minimise alarm fatigue for caregivers, and the smooth glass surfaces, rounded edges and special surface material of the monitors and displays support cleaning and disinfection.
In addition, the MX750 and MX850 monitors comprise of updated features such as enhancements to monitor and evaluate clinical and network device performance, and additional functionalities to improve cybersecurity.
Philips monitoring and analytics general manager Peter Ziese said: “This FDA EUA for our MX750 and MX850 monitors and IntelliVue AD75 and AD85 Active Displays allows us to do that for these remote patient monitoring solutions, which are of vital need in the ICU.
“At Philips, being able to provide the right information at the right time to caregivers has always been a top priority. Now more than ever, there’s an urgent need to make sure those on the frontline have all the available resources at their disposal.”
In May, Philips secured 510(k) clearance from the FDA for its next-generation wearable biosensor to better manage confirmed and suspected Covid-19 patients in the hospital.
