The transaction will help advance LumiraDx’s portfolio of more than 30 assays across common health conditions
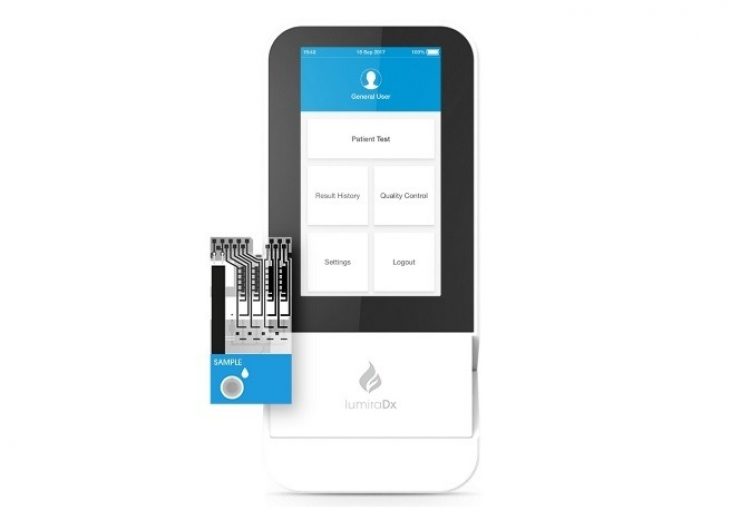
LumiraDx platform and test strip. (Credit: Business Wire)
UK-based next-generation point of care (POC) diagnostics testing company LumiraDx has announced a merger with special purpose acquisition company CA Healthcare Acquisition (CAHC) in a deal worth $5bn.
Under the deal, all current LumiraDx shareholders will hold their existing holdings in the combined firm.
Established in 2014, LumiraDx is engaged in the development, manufacturing and commercialisation of advanced point of care diagnostic platform.
LumiraDx’s diagnostic testing platform, which is under development since 2014, is designed to provide a range of tests that can deliver results within 12 minutes at the point of care.
The deal will help advance LumiraDx’s portfolio of over 30 assays across common health conditions including infectious disease, cardiovascular disease, diabetes, and coagulation disorders.
The company has developed its high sensitivity antigen test for Covid-19 on the LumiraDx Platform. At present, it is being used by the National Health Service (NHS) and Boots in the UK, CVS Health in the US and multiple accident and emergency rooms in Italy and other parts of Europe.
LumiraDx COVID-19 antigen test, which secured CE mark, has also achieved emergency use authorisation (EUA) from the US Food and Drug Administration (FDA).
LumiraDx platform also consists of point of care tests for Covid-19 antibody, INR and D-Dimer. All these tests have secured CE mark and are commercialised in Europe.
It enables to incorporate the commonly used assay technologies such as enzyme, immunoassay, molecular and electrolytes, in addition to sample types such as swab, saliva, and blood.
The multi-channel and low-cost test strips facilitate accurate control and optimisation of each test.
LumiraDx chairman and CEO Ron Zwanziger said: “LumiraDx is at the tipping point of driving a transformation in diagnostic testing. This new public recognition will solidify our already growing presence in the point of care testing market.”
Since its establishment, LumiraDx has pooled $700m in equity capital from investors such as Morningside Ventures, U.S. Boston Capital, The Bill & Melinda Gates Foundation, Petrichor Healthcare Capital Management and other global strategic partners.
