The French start-up's device for interventional oncology will more accurately detect tumours and improve patient outcomes, says the University of Wisconsin
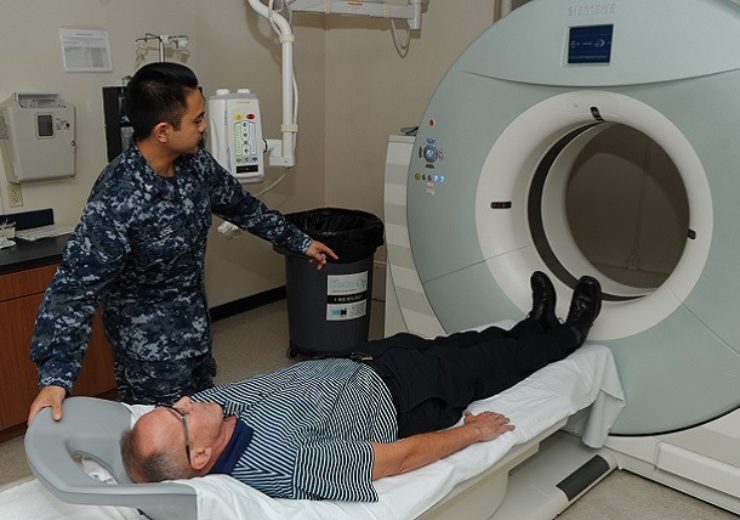
Imactis says its oncology device exposes patients and the healthcare professional performing the procedure to less radiation than regular Computed Tomography (CT) screening (Credit: health.mil)
The University of Wisconsin’s medical centre will become the first US hospital to use Imactis’ image-guided interventional oncology device to improve accuracy in detecting tumours and cut treatment times.
UW Health says the CT-Navigation system, which allows a radiologist to remotely and precisely guide a needle through the body, will help enhance its position in the field of cancer treatment.
It also provides a method of interventional oncology — minimally invasive procedures for the diagnosis or treatment of cancers — that reduces exposure to radiation for both healthcare professionals and patients.
Biopsies and tumour ablations are among the interventional procedures that can be performed using CT-Navigation.
Imactis developed the Computed Tomography (CT) system to allow healthcare professionals plan the trajectory of a needle before guiding it in real-time — making it easier to avoid major organs.
The company says its system requires minimal set-up time, involves a short learning curve and allows even particularly complex procedures to be completed more quickly.
University of Wisconsin professor of radiology and chief of abdominal intervention Dr Fred Lee said UW Health is “very excited” to add this technology.
He added: “It will allow our team to better target and treat lesions [tumours] for ablations, biopsies and many other image-guided interventional procedures.
“This is yet another tool we have that will improve patient care and treatment outcomes while decreasing radiation dose to the operator.”
CT-Navigation – how Imactis’ interventional oncology system works
Before a needle is inserted into the patient’s body, Imactis’ software system can be used to display its anticipated trajectory using a locating device.
Once the optimal trajectory into the patient’s skin has been selected by the physician, a NaviKit — the single-use navigation device for precise insertion of the needle — allows one or more needles to be guided towards the target area.
Imactis claims this means otherwise risky procedures such as biopsies, tumour removals and osteosynthesis — fixing an internal bone fracture — become simpler, safer, and more predictable.
CT-Navigation allows multi-probe procedures involving multiple needles, where precision is key, to be performed more accurately and avoid critical structures including vital organs.
The needle’s progress is continually monitored as it moves through the patient’s anatomy, with new and updated scans being sent to the physician’s screen at any time, allowing them precise control over the procedure.
The CT-Navigation system is both CE-marked and FDA-authorised, making it an approved medical device in both Europe and the US.
It has been installed in 50 hospitals across Europe and has already been used to perform more than 6,000 interventions.
