LipoGlo attaches glowing enzymes to bad-cholesterol particles for researchers to track how they accumulate in fish.
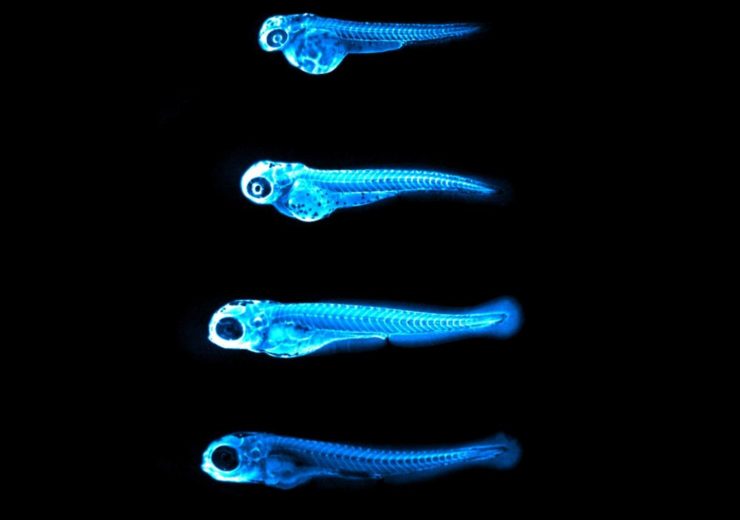
The glowing enzyme attached to the bad cholesterol particles enabled researchers to visualise how much cholesterol is present in each fish, and where in the body it resides (Credit: James Thierer)
The LipoGlo system, created by researchers at the Carnegie Institution for Science, Johns Hopkins University and the Mayo Clinic can help cholesterol management by monitoring the real-time movement of harmful ApoB proteins.
Fat molecules, also called lipids, are passed around the circulatory system by ApoB – with the combination commonly referred to as bad cholesterol.
A dangerous build-up of plaque can then be formed from the bad cholesterol sticking to the lining of blood vessels.
James Thierer, graduate student at Johns Hopkins’ Carnegie Department of Embryology, said: “These ApoB-containing lipoproteins [bad cholestorol] are directly responsible for creating plaques in blood vessels, so learning more about them is essential to fighting the global epidemic of cardiovascular disease.”
How the LipoGlo system can help with cholesterol management
Identifying ways to lower levels of plaque forming in arteries will save lives, but ApoB is a large protein, which makes it difficult to study using traditional molecular biology research techniques.
Scientist Steven Farber from Carnegie leveraged “state-of-the-art” genome editing to track ApoB with a glowing enzyme that’s similar to the one that “lights up fireflies”.
This technique allowed researchers to observe how cholesterol moves, accumulates and gathers to build plaque in real-time and in samples as small as a “near-microscopic droplet of blood”.
Farber said: “Our LipoGlo system allows us to study ApoB in a tiny larval zebrafish, enabling us to try thousands of potential pharmaceuticals and to find the needle in a haystack that could be the next treatment for this terrible disease.
“This type of whole-animal screening is not possible in any other vertebrate.”
The research is being used to find new drugs to fight cardiovascular disease.
Currently, doctors in the US determine the risk of plaque building up inside arteries by measuring the blood concentrations of lipoprotein components such as fat and cholesterol and supplying drugs to slow or even reverse the effects.
However, this is an unreliable treatment that can, in some cases, underestimate the risks and lead to the need for aggressive treatment such as surgical procedures if a build-up of severe symptoms or blockage that threatens muscle or skin tissue survival is found.
“Statin drugs have helped a lot of people and saved many lives, but folks still die of cardiovascular disease every year, so there is an urgent need for new medical strategies to understand and prevent arterial plaque build-up,” Farber said.
Using the LipoGlo system, the team of researchers also discovered a gene called pla2g12b, which had a huge impact on the size and number of ApoB-containing lipoproteins in the zebrafish.
Farber said: “Although there is much more work to be done to fully understand the processes underlying atherosclerosis, these findings show that our LipoGlo tool has the power to transform our understanding of lipoprotein biology, which will have important implications for future strategies to treat heart disease.”
