The ceramic implant ZERAMEX XT offers clinicians a metal-free alternative supporting natural, esthetics, expanding Glidewell Dental implant portfolio
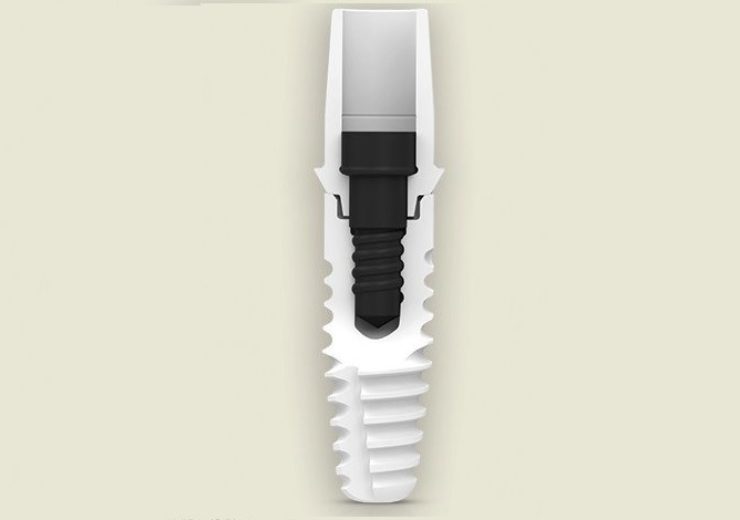
Image: Glidewell Dental Launches ZERAMEX? XT Implant. Photo: Courtesy of Glidewell Dental.
Glidewell Dental has launched a new two-piece ceramic, metal-free implant ZERAMEX XT at the recently held 2019 Glidewell Dental Symposium in Orlando, Florida.
As the newest addition to its line of implant products and services, ZERAMEX XT is claimed to have been designed to help clinicians provide lifelike esthetics for cases in the anterior or with thin tissue biotypes.
Glidewell Dental claims that its ZERAMEX XT implant is 100% metal-free and highly biocompatible, offering high degree of restorative flexibility compared to single-piece ceramic implants.
Glidewell Dental chief operating officer Greg Minzenmayer said: “The addition of ZERAMEX XT to our implant portfolio is part of our ongoing efforts to help dentists meet the varying needs of their patients.
“The implant is a great, metal-free alternative for cases with high-esthetic demands, and we consider it yet another simple, accessible solution for expanding implant services in the general practice.”
Glidewell Dental’s Zirconia implants have low affinity for plaque adhesion
Glidewell Dental also claims that Zirconia implants can be advantageous from not only esthetic viewpoint but are also hygienic, in the form of low affinity for plaque adhesion.
The implant includes a new platform-switched internal connection that supports an expanded range of restorative protocols.
The company claims that the ZERAMEX XT also features a beveled platform that eases the placement of prosthetic components, a ‘Bolt-In Tube’ connection that minimizes the transmission of forces to the implant, four interlocks offering anti-rotation protection and VICARBO screw manufactured from carbon fiber-reinforced polymer.
The root-shaped design of the implant is also claimed to help achieve high stability, while its hydrophilic-treated Zerafil surface supports better osseointegration.
Available in 4.2 mm and 5.5 mm diameters, the implant and the system includes an array of prosthetic components to support a full range of temporary and permanent restorative protocols.
