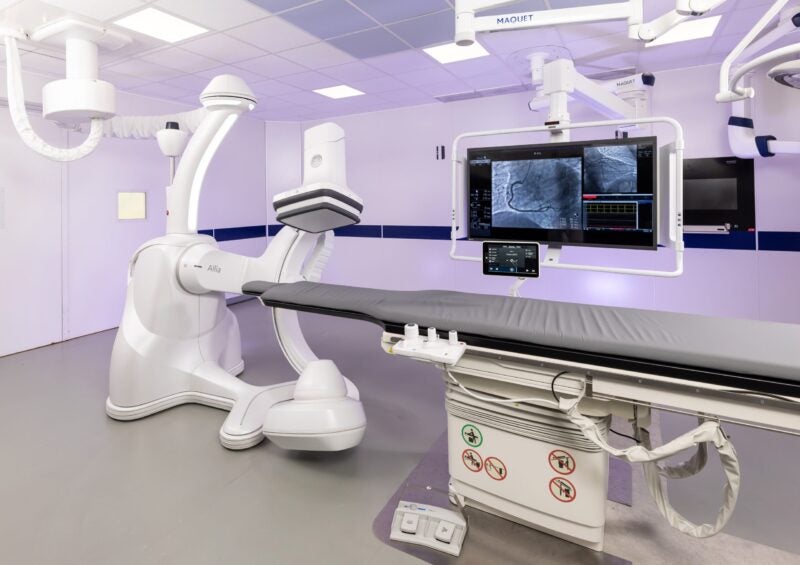
GE HealthCare has obtained 510(k) clearance from the US Food and Drug Administration (FDA) for Allia IGS Pulse image-guided system (IGS) for improved cardiac imaging.
Allia IGS Pulse, the latest addition to the US-based company’s IGS portfolio, is designed to improve workflow to diagnose and treat cardiovascular diseases in interventional cardiology.
It is said to have a new imaging chain designed to offer imaging at the right dose for visible impact in complex cardiology interventions irrespective of patient size.
Built on the Allia platform, the IGS provides a customised workspace that meets the operator’s specific needs and preferences, according to GE Healthcare.
Under the new image chain, the system has the first monopolar X-ray tube that is being used to take pictures of interventional procedures.
This tube will improve the operating room environment during a procedure as it is stronger and quieter than a typical conversation.
Even with 30cm detector setups, the new tube’s compact footprint enables doctors to achieve steep angulation for a better understanding of coronary artery anatomy, the medical technology firm said.
The system’s most recent integration of MyIQ technology enables doctors to customise their experience without adding more doses by selecting their preferred image look from four different image types with just one click.
GE HealthCare interventional general manager Arnaud Marie said: “I’m excited by the addition of Allia IGS Pulse to our interventional offerings because it addresses the very things clinicians continue to tell us present challenges in their day-to-day practice.”
Allia IGS Pulse can help interventionalists obtain superior image quality for big and obese patients with a BMI of greater than 30.
Its reduced pulse width and X-ray peak power minimise the motion blur to improve the visibility of moving objects like devices and vessels.
With AutoRight PLUS, the company’s next-generation automation technology, Allia IGS Pulse can help optimise picture quality and dose during treatments.
The system now optimises seven parameters, including Focal Spot Shape, in real-time using AutoRight PLUS, the medical technology company added.
Additionally, a set of tools is also offered to enhance dosage efficiency, dose reduction, and dose awareness to further optimise dose along the image chain.
Allia IGS Pulse has been in pilot operations since January 2023 at Clinique Pasteur – Toulouse in Toulouse, France.





