The Evoque system, which comprises a nitinol self-expanding frame, intra-annular sealing skirt and tissue leaflets made from bovine pericardial tissue, is offered in three different sizes, all delivered through the same low-profile transfemoral 28F system
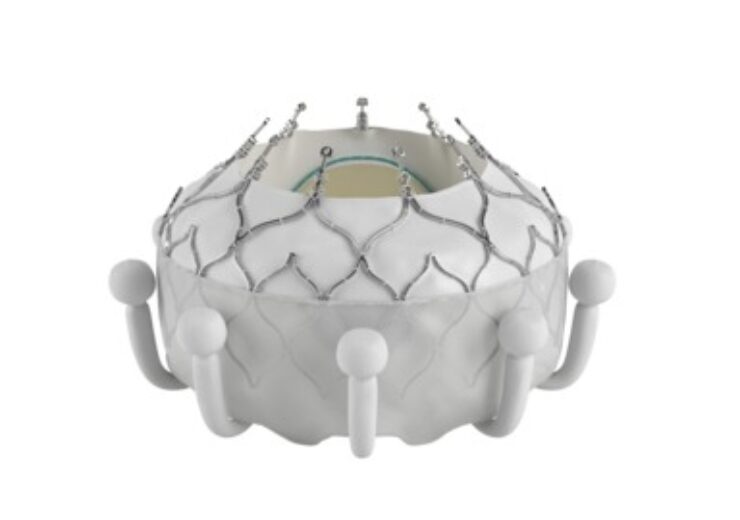
Edwards’ Evoque tricuspid valve replacement system. (Credit: Edwards Lifesciences)
US-based medical technology company Edwards Lifesciences has received the US Food and Drug Administration (FDA) approval for its Evoque tricuspid valve replacement system.
The Evoque system comprises a nitinol self-expanding frame, intra-annular sealing skirt and tissue leaflets made from the company’s tested bovine pericardial tissue.
Edwards is offering the tricuspid valve replacement system in three different sizes, all of which are delivered through the same low-profile transfemoral 28F system.
The system is indicated for improvement of health status in patients with symptomatic severe TR despite optimal medical therapy (OMT), who are eligible for valve replacement procedures.
The FDA approval for the Evoque tricuspid valve replacement system follows a CE Mark approval, granted in October last year.
Evoque is the first valve replacement therapy to receive FDA approval for the treatment of tricuspid regurgitation (TR), said the US medical technology company.
Edwards transcatheter mitral and tricuspid therapies corporate vice president Daveen Chopra said: “Edwards has a long history of leading innovation and pioneering new therapies to address the unmet needs of patients with structural heart disease.
“We are grateful for the strong collaboration with clinicians all over the world who contributed to the Evoque system now being available through FDA’s Breakthrough Pathway to provide a treatment option to the many patients in the US suffering with tricuspid valve disease.”
The six-month results from the randomised controlled trial, dubbed TRISCEND II, the Evoque system showed favourable safety and effectiveness, meeting all primary endpoints.
In the study, the system showed a significant reduction of tricuspid regurgitation and sustained quality of life improvement, while showing a favorable balance between risk and benefit.
The study results showed favourable trends in the device group compared to the control group in the primary composite endpoints.
The endpoints include all-cause mortality, tricuspid intervention, heart failure hospitalisation, KCCQ, NYHA and 6MWD.
TRISCEND II study principal investigator Susheel Kodali said: “Patients suffering with tricuspid regurgitation endure life-impairing symptoms and, until today, had no approved transcatheter treatment options.
“The Evoque system can replace the native tricuspid valve, virtually eliminating tricuspid regurgitation in a wide range of patients.
“We see significant improvements in patients’ symptoms and quality-of-life, including not feeling short of breath and being able to care for themselves, which ranked highest on a patient preference survey conducted at baseline with TRISCEND II pivotal trial patients.”
