ControlRad Trace is the only technology that can be integrated into existing mobile C-arms to reduce radiation in any fluoroscopic imaging procedure
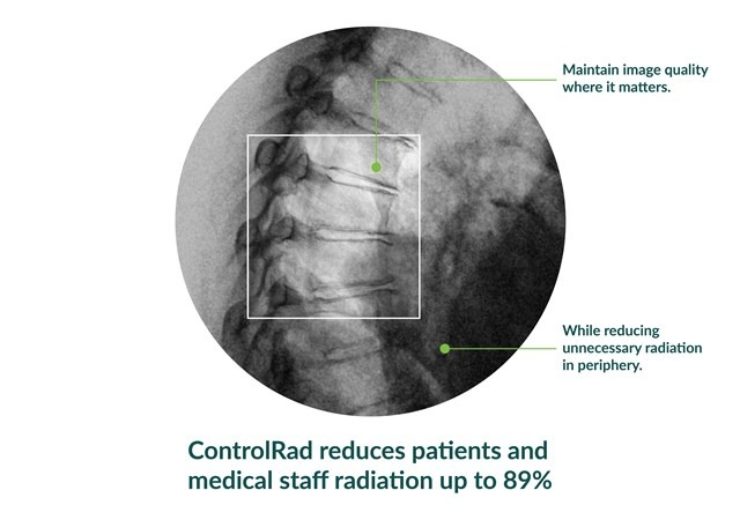
Image: ControlRad Trace system will reduce radiation in any fluoroscopic imaging procedure. Photo: courtesy of Business Wire.
Medical technology firm ControlRad has secured $15m series B funding to advance the commercial launch of its ControlRad Trace system.
The series B financing round was led by Questa Capital. The medical device industry veterans Ryan Drant and Tim Patrick have also joined ControlRad as the board of directors.
ControlRad CEO Guillaume Bailliard said: “The investment from Questa Capital and the additions of Ryan and Tim to the board will be invaluable as we begin the launch of ControlRad Trace and build out our commercial organisation.
“Both have proven track records of launching innovative technologies and building great teams.”
ControlRad Trace technology to minimise radiation in any fluoroscopic imaging procedure
ControlRad Trace is claimed to be the first FDA-cleared technology, which can be incorporated into existing mobile C-arms to minimise radiation in any fluoroscopic imaging procedure (FGP).
In May this year, the company secured FDA 510(k) clearance for the ControlRad Trace technology.
The system holds the capacity to decrease radiation exposure by up to 89%, enabling to protect patients and medical staff without disturbing image quality or workflow.
ControlRad Trace solution, which has an advanced semi-transparent filter, tablet and image processing technology, can be can be retrofitted on existing C-arms.
The medical staff can draw a region of interest (ROI) on a ControlRad tablet, which will help optimise image quality in real-time in the ROI and reduce unnecessary radiation in the periphery.
Questa Capital founder and principal Ryan Drant said: “The opportunity to invest in ControlRad highlights our commitment to funding and supporting technologies that will have an immediate impact on the health and wellness of patients and medical staff.
“Guillaume and the team have made enormous progress recently with regulatory clearance and initial clinical use.”
Based in Atlanta of Georgia, ControlRad is involved in the development of advanced products for the reduction of radiation exposure from FGP for patients and healthcare professionals.
