The microneedling device has been cleared to enhance the look of facial acne scars in Fitzpatrick skin types 1, 2, and 3 in patients of age 22 years and older
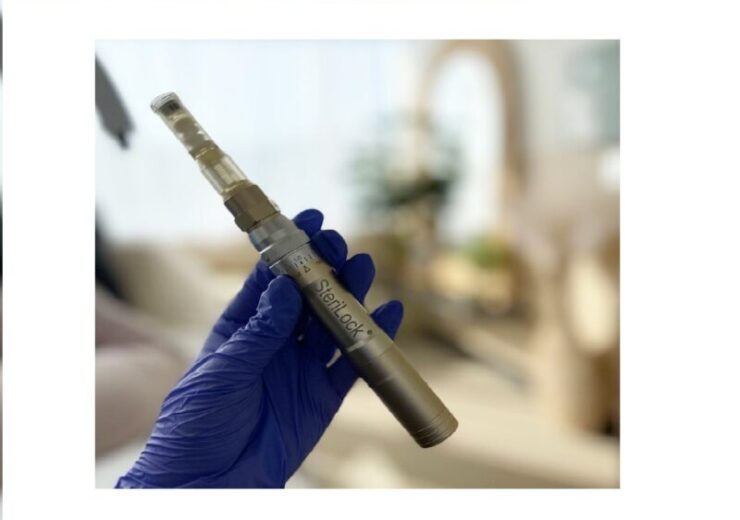
The SkinStylus SteriLock MicroSystem from Beauty Health (Credit: Business Wire)
US-based Beauty Health has received clearance from the US Food and Drug Administration (FDA) for the use of its SkinStylus SteriLock MicroSystem on facial acne scarring.
The microneedling device has been cleared to enhance the look of facial acne scars in Fitzpatrick skin types 1, 2, and 3 in patients of age 22 years and older.
It is intended to provide a minimally invasive treatment by using small needles to make tiny, controlled micro-injuries. This helps activate the production of collagen and elastin for smoother, firmer, and even-toned skin.
SkinStylus SteriLock MicroSystem is now said to be the only FDA-cleared microneedling device for both the face and abdomen.
Beauty Health, which owns the Hydrafacial brand, said that the approval is a key milestone for SkinStylus’ growth. The brand was acquired by the Nasdaq-listed skin care company in February 2023.
Beauty Health president and CEO Andrew Stanleick said: “SkinStylus’ new facial indication for acne scarring is a testament to Beauty Health’s innovation track record, proven brand building expertise, and our commitment to creating the future of skin health,”
“Our goal is to become the world’s leading beauty, health and wellness platform by bringing together incredible brands and growing them into category leaders.
“This SkinStylus indication further unlocks the potential of the highly complementary treatments in our multi-brand ecosystem and positions SkinStylus for exponential growth in the years ahead.”
SkinStylus is an aesthetician-founded brand, and the SkinStylus SteriLock MicroSystem has a set of features for medical and aesthetic professionals.
The SkinStylus SteriLock MicroSystem is already classified by the FDA as a Class II Medical Device.
SkinStylus’ FDA clearance for the treatment of surgical or traumatic hypertrophic scars on the abdomen is for adults aged 22 years and over. The clearance was based on the clinical findings that the skincare company presented to the US health regulator.
