The new investment is expected to help the company produce more than 12 million Covid-19 diagnostic test kits per month by the end of February 2021
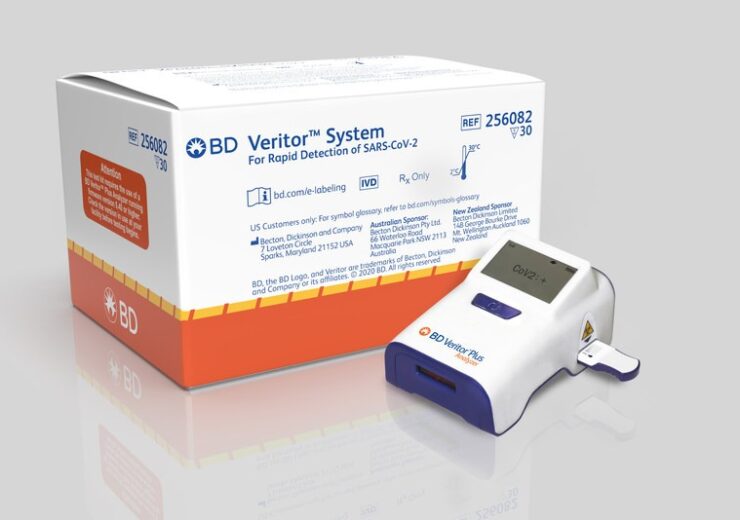
BD Veritor Solution for Rapid Detection of SARS-CoV-2. (Credit: BD (Becton, Dickinson and Company).)
Becton, Dickinson and Company (BD) has received $24m investment from the US government to ramp-up the manufacturing of its BD Veritor Solution for rapid detection of SARS-CoV-2.
The US Department of Defence, in collaboration with the US Department of Health and Human Services has offered the funding, which is expected to help the company produce more than 12 million test kits per month by the end of February 2021.
BD integrated diagnostic solutions president Dave Hickey said: “Making COVID-19 diagnostic tests widely available is critical to expanding rapid detection of COVID-19 infections, and mitigating the impact of the disease by identifying affected patients, quickly quarantining infectious individuals and tracing their contacts.
“This investment will bolster our U.S. manufacturing capabilities helping us quickly scale our production of point-of-care COVID-19 tests to ensure we have a robust supply for our U.S. customers.”
BD would deploy the new assay across its installed BD Veritor Plus device in the US
The BD Veritor Plus SARS-CoV-2 antigen assay has been granted the FDA emergency use authorization on 6 July 2020, for use by authorised laboratories.
BD intends to deploy the new SARS-CoV-2 assay across more than 25,000 BD Veritor Plus instruments installed in the US.
The US-based medical technology company has designed the device to be around size of a cell phone, for easy use in different clinical settings such as hospitals, clinician offices, urgent care centers, and retail pharmacies.
The BD Veritor Plus System for Rapid Detection of SARS-CoV-2 Assay is a CLIA-waived immunoassay, intended for use in health care settings, to rapidly detect Covid-19 in symptomatic individuals.
The company has conducted clinical studies at more than 20 centres across the US, demonstrating that the diagnostic test can achieve 84% sensitivity and 100% specificity.
The test has been authorised by the FDA only for the detection of proteins from SARS-CoV-2, but not for any other viruses or pathogens.
