Baxter International has secured 510(k) clearance from the US Food and Drug Administration (FDA) for PrisMax system and integrated TherMax blood warmer, the next-generation platform for continuous renal replacement therapy (CRRT) and therapeutic plasma exchange (TPE).
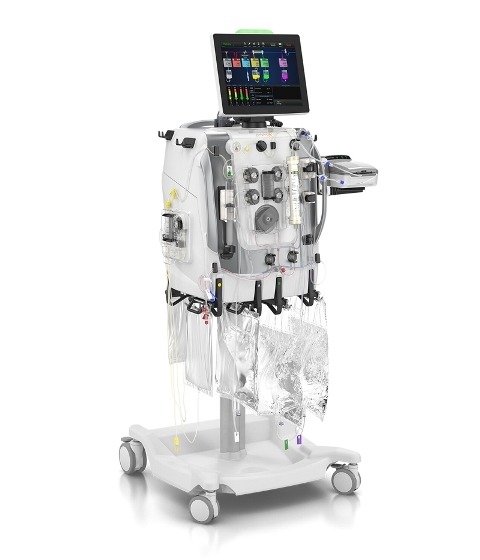
Image: The PrisMax system and integrated TherMax blood warmer is Baxter's next-generation platform for continuous renal replacement therapy and therapeutic plasma exchange. Photo: courtesy of Business Wire.
Developed using suggestions from more than 650 healthcare providers across the world, the PrisMax system will enable to simplify therapy delivery and provides flexibility to the hospitals to meet the unique demands of the ICU.
PrisMax system, which is designed based on Baxter’s Prismaflex technology, will be used for the treatment of patients with acute kidney injury (AKI) and select autoimmune diseases.
The rate of Medicare fee-for-service beneficiaries experiencing a hospitalisation complicated by AKI doubled between 2006 and 2016, as per the United States Renal Data System (USRDS),
PrisMax is provided with new digital health features, which enable hospitals to connect the system to electronic medical record (EMR) platforms. It allows direct integration of information from PrisMax to the EMR to help ICU nurses to spend less time manually for the documentation of treatment data and reduce the risk of transcription errors.
The system also features TrueVue Analytics, Baxter’s advanced data and analytics platform. Hospitals will use TrueVue Analytics tools for the assessment of data aggregated at the hospital level and evaluate the quality and effectiveness of their CRRT programmes.
A prospective and multicentre international pilot study demonstrated that PrisMax delivered significant improvements in areas that impact efficiency and ease of use, including the time needed for bag changes, the number of informational and malfunction alarms.
Designed to use exclusively with the PrisMax system, the TherMax blood warmer is a major component for extracorporeal CRRT to warm the blood prior to returning to the body, allowing to keep the patient’s body temperature at a normal level
The company has introduced PrisMax in over 20 countries across Europe and Australia, and is planning to submit applications for regulatory approval of the system in additional countries in this year and 2020.
Baxter’s US renal business general manager Gavin Campbell said: “We looked at every detail during the PrisMax design process. Our team reviewed every piece of feedback from nephrologists, nurses and intensive care specialists, and then designed a system that can help simplify therapy administration and maximize efficiency.
“We put our 20 years of expertise in CRRT and blood filtering technology to work to design an advanced system that allows for clinicians to customize treatment parameters to meet the needs of their patients.”
