FDA approval allows Perfusor Space, Infusomat Space, and Outlook ES Pumps for tracheal delivery of continuous nebulized medications into a nebulizer
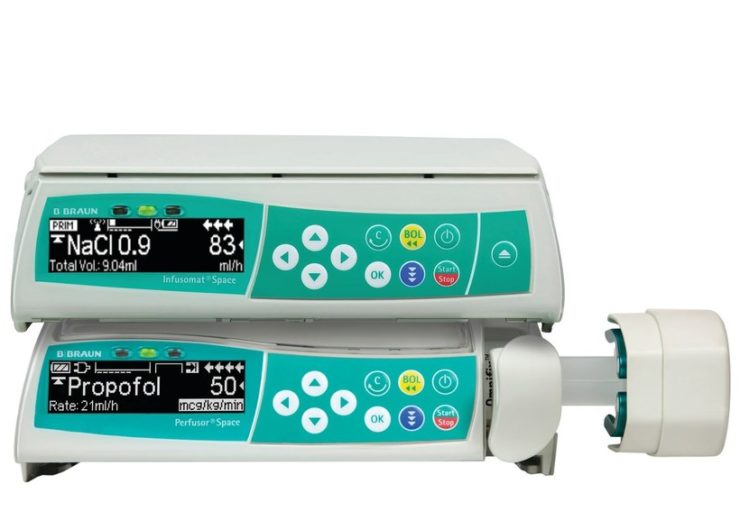
2nd Generation Infusomat Space Infusion Pump and Perfusor Space Syringe Pump. (Credit: B. Braun Medical.)
Germany-based pharmaceutical devices firm B. Braun Medical (B. Braun) has secured the US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) for its infusion pumps to treat patients with COVID-19.
The FDA authorization allows the company’s Perfusor Space Syringe Infusion Pump, Infusomat Space Volumetric Infusion Pump, and Outlook ES Pump systems for tracheal delivery of continuous nebulized medications into a nebulizer to treat COVID-19 patients and decrease.
B Braun scientific affairs chief medical officer and senior vice president Wes Cetnarowski said: “This authorization allows for an alternative method to administer continuous nebulized medications to patients who are critically ill with COVID-19, many of whom are on ventilation.
“As hospitals struggle to cope with the surge of patients suffering from this deadly disease, this action provides another tool for healthcare professionals on the front line to treat some of the most serious cases while helping to protect clinicians by reducing their exposure to infected patients.”
Perfusor Space Syringe Infusion Pump System are approved for ground transport
According to the previous studies, using infusion pumps with nebulizers would deliver steady and controlled nebulized medication to patients with acute respiratory distress syndrome (ARDS).
The company said that some of most critical COVID-19 patients suffer from severe ARDS.
B. Braun said that the Perfusor Space Syringe Infusion Pump System is already cleared for ground transport, and the recent EUA allows the medical transport of the Infusomat Space Volumetric Infusion Pump System.
The company submitted the EUA request to FDA on 8 April 2020.
B. Braun Medical chairman and chief executive officer Jean-Claude Dubacher said: “We applaud this decisive action taken by the FDA to help some of the most seriously ill COVID-19 patients.
“The rapid review and authorization of this and other COVID-19 countermeasures demonstrate the agency’s commitment to ensure that healthcare providers have the medical devices and treatments they need to fight this disease.”
