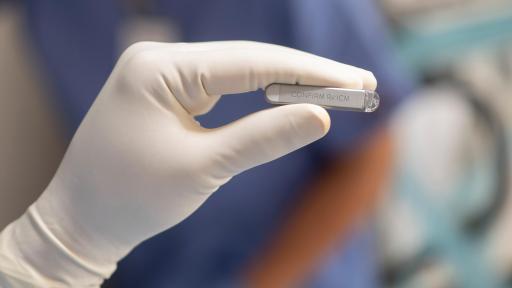
The Confirm Rx ICM is a paperclip-sized implantable device, which aggregates smartphone connectivity and continuous, remote monitoring to track unpredictable heart rhythm problems for rapid and precise diagnosis.
Abbott already secured CE mark approval and the US Food and Drug Administration (FDA) clearance for the next-generation Confirm Rx ICM device.
An arrhythmia is an abnormal heart rhythm, which may result in symptoms such as the feeling that the heart is racing, dizziness, shortness of breath or fainting.
The Confirm Rx ICM device will help physicians to remotely monitor arrhythmias in people most at risk.
The device, which will be inserted just under the skin in the chest above the heart through minimally invasive outpatient procedure, is claimed to be only ICM that can sync to a smartphone via Bluetooth and transmit information to the physician to help detect irregular heartbeats rapidly.
Abbott’s mobile app helps to avoid the use of an additional transmitter, and is claimed to be user-friendly health technology translated in around 40 languages.
Abbott cardiac rhythm management therapies medical director Dr Avi Fischer said: “Through new advances like Abbott’s next generation of Confirm Rx ICM, physicians can act more proactively and efficiently in their treatment approach, and patients can stay engaged and connected.”
Confirm Rx ICM features new detection technology, which will help decrease false detection by 97% and avoids alerting physicians of irregular heartbeats that may not actually signal a cardiac arrhythmia.
The device also integrates new SharpSense technology, which was designed with physician feedback and insights from the people who have benefited from the Confirm Rx ICM.
In March this year, Abbott secured an additional indication from the FDA for its MitraClip clip delivery system to treat certain heart failure patients with mitral regurgitation.
Abbott Vascular’s device can be used for the treatment of moderate-to-severe or severe mitral regurgitation, which is a leakage of blood backward through the mitral valve into the heart’s left atrium.




