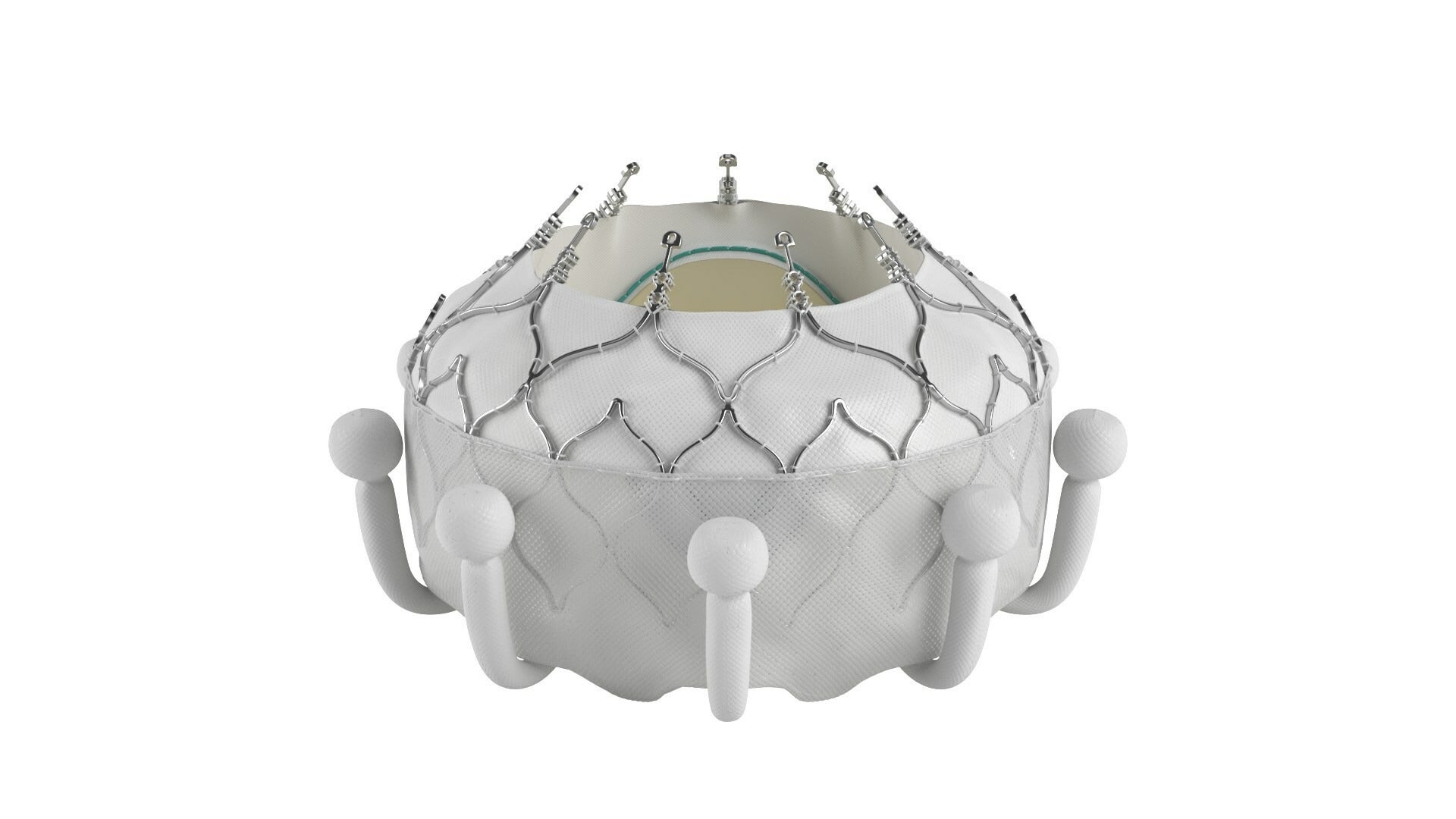
Edwards Lifesciences has received the CE mark approval for its EVOQUE tricuspid valve replacement system to treat eligible patients with tricuspid regurgitation (TR).
The EVOQUE system comes with a nitinol self-expanding frame, intra-annular sealing skirt, and tissue leaflets made from the same bovine pericardial tissue as the company’s heart valves.
Edwards is offering the EVOQUE valves in three different sizes, all delivered through a low-profile transfemoral 28F system.
The EVOQUE system is the world’s first transcatheter valve replacement therapy to receive regulatory approval to treat TR, said the US medical technology company.
Edwards Lifesciences transcatheter mitral and tricuspid therapies corporate vice president Daveen Chopra said: “Innovating for unmet patient needs is at the centre of everything we do at Edwards, which makes us especially proud to have received CE Mark for this first-of-its-kind transcatheter tricuspid valve replacement therapy.
“With the EVOQUE system’s approval, in addition to our current PASCAL tricuspid system, we are now able to provide a broader array of much-needed treatment options for appropriate tricuspid disease patients in Europe.”
The EVOQUE system has been studied in a single-arm, global, multi-centre study, TRISCEND.
In the TRISCEND study, patients treated with the EVOQUE system showed favourable safety and effectiveness outcomes and significant quality-of-life improvements after one year.
The system resulted in a high survival rate of 90.1% and high freedom from heart failure hospitalisation of 88.4%, with a significant and sustained TR reduction of 97.6%.
It also significantly improved the functional and quality-of-life outcomes in 93% of patients compared to 26% at baseline and a 26-point increase in KCCQ score over baseline.
Edwards has also secured the CE mark for a portfolio of transcatheter therapies, including the PASCAL Precision transcatheter repair system and the Cardioband annular reduction system.
TRISCEND II study European principal investigator Philipp Lurz said: “The EVOQUE system is able to fully replace the tricuspid valve, virtually eliminating tricuspid regurgitation in a wide range of anatomies.
“The significant improvements in patients’ quality-of-life are remarkable, now offering a therapy to many patients who previously had no treatment options.”



