
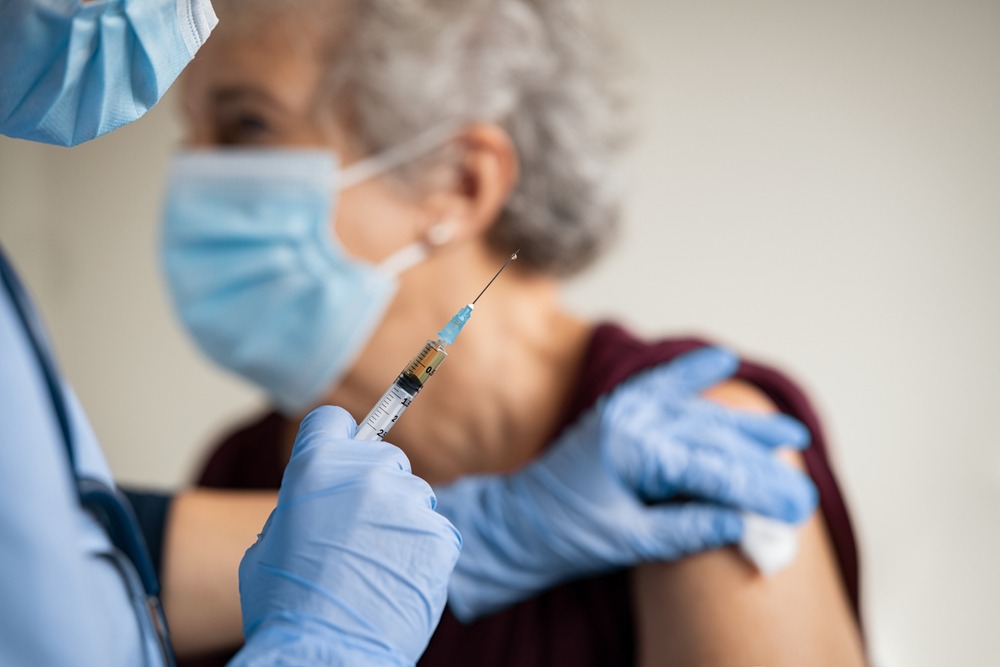
Over the past year, American biotech firm Vaxess Technologies has moved to the forefront of one very specific part of global efforts to tackle the Covid-19 pandemic – developing a refrigeration-free, virtually painless vaccine patch.
The company was founded by a small group of Harvard University graduates in 2011 with the intent to develop more accessible and effective vaccine products for a range of infectious diseases.
Vaxess started out working on a vaccine patch for influenza – which is placed on the skin and, through dozens of tiny silk microneedles, gradually releases antigens into the body.
At the beginning of 2020, however, the Massachusetts-based firm pivoted its work towards the coronavirus pandemic and has since achieved several major milestones relating to these efforts.
The proprietary MIMIX technology Vaxess’ single-dose skin patch is based on means it does not require refrigeration, and can therefore be conveniently ordered, shipped and applied at home by patients.
It is also designed to be removed and then easily disposed of after only a few minutes – at which point microneedles embedded in the skin continue to deliver immunisation therapy over several weeks.
The company believes this long list of potential benefits means its novel patch could yet play a key role in vaccinating populations against the virus globally.
From government contracts to major industry partnerships, as well as the distinct possibility of groundbreaking clinical trials later this year, we take a closer look at Vaxess’ journey so far.
Vaxess timeline: Developing a Covid-19 vaccine patch
Pre-pandemic foundations
Prior to 2020, several financial investments helped put Vaxess in a strong position to take its MIMIX technology forward once the Covid-19 health crisis struck.
In April 2015, the company received two separate grants from the US National Science Foundation (NSF) and the National Institute of Health (NIH), totalling $360,000, which it put towards developing “novel heat-stable rotavirus and polio vaccines, and advancement of innovative vaccine presentation formats”.
Having gained two further NIH grants the previous year, Vaxess secured its most significant cash injection to date in March 2017 – a $6m award from the Bill & Melinda Gates Foundation to further its work in microneedle-based vaccines patches.

In June 2019, the company was able to close an $8.2m Series A funding round led by The Engine, a venture capital firm launched by MIT to invest in early-stage tech innovators, bringing its total grant and equity funding to more than $22m.
October of that year also saw Vaxess begin collaborating with leading influenza vaccine manufacturer GC Pharma to further progress on its smart-release patch technology, with the South Korean drug company obtaining future rights to market the product in its home country.
Shifting focus to Covid-19
In late-August of last year, Vaxess was awarded a federal contract by BARDA (Biomedical Advanced Research and Development Authority), as part of the US governmental department’s efforts to evaluate alternative vaccination technologies.
BARDA provided a total of about $2.5m to three organisations working on skin patch technologies – the University of Connecticut, Verndari and Vaxess – and one named Esperovax that is currently developing an orally-administered Covid-19 vaccine pill.
Vaxess’ share of this funding, which reportedly amounted to $749,000, was put towards integrating vaccine material into its MIMIX platform, as well as demonstrating the feasibility of the skin patch as a vaccination method.
At this point, the company had begun working towards a “combination seasonal protection patch” including both a novel coronavirus vaccine and a seasonal flu vaccine.
In a statement at the time, Michael Schrader, CEO and co-founder of Vaxess, said: “By supporting development activities in parallel, BARDA has helped advance first-generation Covid-19 vaccines through clinical development remarkably fast with a focus on safety and effectiveness.
“We’re thrilled to see them expanding their focus to include innovative platforms like MIMIX that can enable even better responses to future pandemics.”
Medigen partnership and progress towards clinical trials
It took less than a month for Vaxess to take its next major step forward, announcing a partnership with Taiwanese pharmaceutical company Medigen on 14 September 2020.
This partnership has seen both companies investigate a combined coronavirus-influenza vaccine candidate that Medigen co-developed with researchers at the Vaccine Research Center of the NIH’s National Institute of Allergy and Infectious Diseases (NIAID), and the University of Texas at Austin.
Medigen entered Phase I clinical trials to assess adjuvanted intramuscular injection of the vaccine later the same month, and – in early-2021 – the company announced plans for Phase II trials following the successful dosing of all participants in the first stage.
To complement these efforts, Vaxess announced it would initiate US clinical trials for delivering the combined vaccine using its MIMIX patch technology.
While the results of these investigations are yet to be revealed, the Massachusetts-based firm has released two new updates so far in 2021 – more funding, amounting to $2m, from the NSF and US Defense Advanced Research Projects Agency (DARPA), and the addition of American businessman Ted Hibben as chief business officer.
Within the second of these announcements, Vaxess also reiterated plans to advance its MIMIX platform to a Phase I proof-of-concept trial “this year” – indicating that, while it will no doubt be some time before we see the company’s vaccine patch become commercially available, these efforts are continuing to move rapidly in the right direction.






