
The Covid-19 pandemic may have had an overwhelming impact on the types of medical devices gaining FDA approval in 2020, but several innovations in the cardiovascular space have also been given regulatory clearance so far this year.
Alongside the numerous diagnostic tests, ventilators, respirators and PPE items that have secured an emergency use authorisation (EUA) to help healthcare providers cope with the virus’ effect, a total of 13 cardiovascular products have been given market approval by the US regulator.
Analytics firm GlobalData had anticipated that the cardiovascular sector would be one of the areas to be hit hardest by the crisis in June 2020 – suggesting revenues may not be recoverable for manufacturers of these heart and blood vessel-related products.
However, with cardiovascular heavyweights Abbott Laboratories and Edwards LifeSciences both releasing relatively positive Q2 earnings reports in July, and a similar number of heart-oriented medical devices already receiving FDA approval this year compared to 2019, the outlook may not be as bleak as previously thought.
We take a look at five of the most innovative devices in this space that have been given FDA clearance so far in 2020.
FDA-approved cardiovascular devices in 2020
Edwards LifeSciences SAPIEN 3 transcatheter heart valve system
California-based medtech giant Edwards LifeSciences gained approval for its SAPIEN 3 transcatheter heart valve (THV) system on 31 August.
The SAPIEN 3 consists of an artificial heart valve, made from cow tissue, and is catheter-based – meaning it can be implanted in the body without the need for open-heart surgery.
Having previously been approved to treat severe aortic stenosis, its latest FDA clearance means it can also be used to treat pulmonary regurgitation, where blood leaks back into the heart, or pulmonary stenosis, where the pulmonary valve narrows and restricts blood flow.
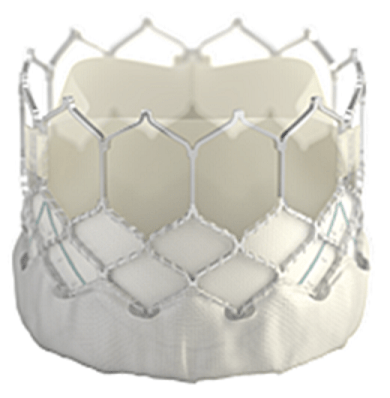
It does this by replacing a failing pulmonary valve in patients born with heart defects, and opening and closing to force blood flow in the correct direction.
The SAPIEN 3 is also equipped with Edwards LifeSciences’ commander delivery system, which allows a doctor to move the valve through a large vein in the patient’s leg, using a catheter, to reach the heart.
By avoiding open-heart surgery – which result in longer recovery times and more scarring – this is intended to give patients more time between open surgeries, or reduce the total number of open surgeries the patient needs.
Cardinal Health multi-function defibrillation electrodes
Ohio-based firm Cardinal Health, which makes anaesthesia products, surgical equipment and patient monitoring tools as well as cardiovascular devices, gained FDA clearance for its multi-function defibrillation electrodes on 7 August.
The electrodes are a pair of disposable pads that, when attached to one of several compatible defibrillator devices, can be used to detect, and help correct, irregular heartbeats in a person experiencing cardiac arrest.
One side of each electrode contains a sensor, and sticks to a person’s chest to measure electrical signals from their heart.
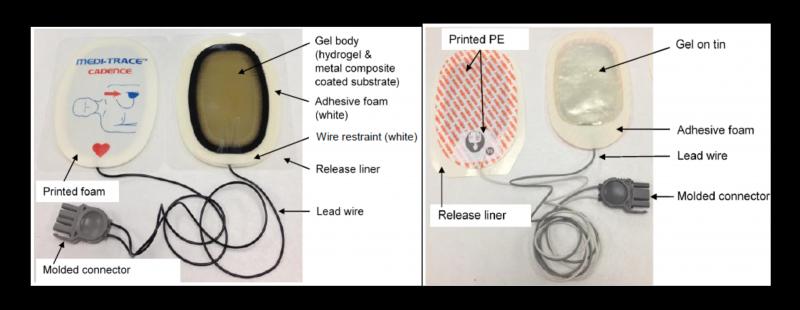
It can then detect irregular heartbeats, and deliver an electrical pulse to restore the heart’s normal beating via the attached defibrillators.
This can reduce the amount of time an unconscious or unresponsive patient spends in cardiac arrest, and ultimately increase chances of survival.
Cardinal Health’s electrode pads are designed for use in both clinical and out-of-hospital settings.
WATCHMAN left atrial appendage closure devices
Massachusetts-headquartered medical device manufacturer Boston Scientific – another giant name within the industry – gained FDA clearance for its WATCHMAN and WATCHMAN FLX left atrial appendage closure devices on 21 July.
While the two devices are slightly different shapes, they both have the same intended use, which is to prevent blood clots in a patient’s left atrial appendage (LAA) – a small pouch found inside the muscle wall of the heart’s left atrium – from entering into their bloodstream.
These blood clots often form in patients experiencing non-valvular atrial fibrillation – an irregular heartbeat that is a result of high blood pressure or an overactive thyroid gland, rather than heart valve disease – and can cause a stroke if they travel through the bloodstream and block a blood vessel in the brain.
The WATCHMAN device is inserted into a patient’s body using a catheter, and permanently implanted in the LAA.
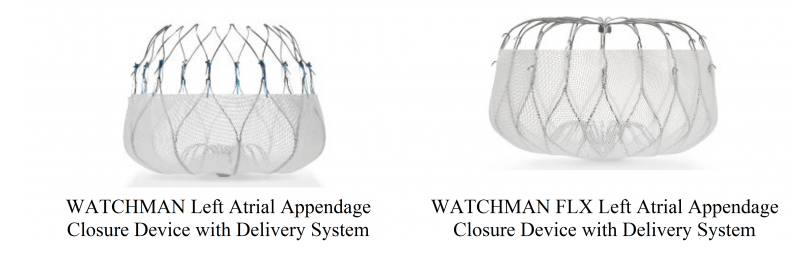
Once it has been implanted, the device opens up like an umbrella, allowing a thin layer of tissue to grow over it and stopping blood clots in the LAA from entering the bloodstream. This tissue growth takes about 45 days in total.
The FDA’s approval in July specifically approved the use of the WATCHMAN devices in patients with non-valvular atrial fibrillation who are at increased risk of stroke, and are recommended and suitable for blood-thinning medication but also have an appropriate reason to seek a non-drug alternative to these medications.
The approval also covers the catheter-based delivery systems used to insert the WATCHMAN devices into a patient’s body.
Philips HeartStart FR3 defibrillator and accessories
Dutch healthcare heavyweight Philips Medical Systems gained regulatory approval for its HeartStart FR3 defibrillator on 11 May.
The device is a battery-powered AED (automated external defibrillator) used to treat patients experiencing a sudden loss of heart function, or cardiac arrest.
It uses two multi-function defibrillation electrodes, or sensors, which are placed on the patient’s chest to detect an abnormal heartbeat, and can deliver a controlled electric shock to correct this.

The defibrillator’s user interface provides voice, text or icon prompts to guide the user through the rescue process associated with cardiac arrest – which can include cardiopulmonary resuscitation (CPR) as well as shock delivery.
As part of the same notification, the FDA also approved several accessories used alongside the device, including a primary battery, rechargeable battery and corresponding charger, and two sets of compatible electrode pads.
It is designed for use in adults, and in children weighing more than 55lbs or over the age of eight – although part of the FDA’s approval covers an optional paediatric key that means certain models can be used to treat children under the age of eight as well.
Alto abdominal stent graft system
The Alto abdominal stent graft system gained FDA clearance on 13 March following an application from Endologix – a California-based medical devices firm that specialises in treating aortic disorders.
The Alto stent graft system is intended to repair an abdominal aortic aneurysm, in which a lower section of the aorta – the main artery supplying blood to the rest of the body – is weakened and bulging.
The stent graft is delivered into a small cut in the upper leg using a catheter and guided by a clinician to the site of the aneurysm, where it expands to fit the width of the aorta.
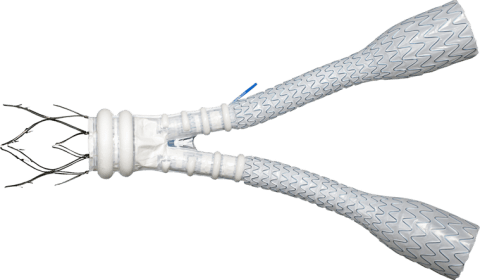
This widens the opening in the blood vessel, providing a path for blood to flow and preventing further aneurysm growth or possible rupture of the aorta.
In a clinical study of patients treated with the Alto stent graft, the FDA reports that 95% of patients – 58 out of 61 – did not have device- or aneurysm-related issues one year on from the procedure.
The system consists of an implant featuring two plastic tubes and a metal framework made from nickel-titanium alloy, extension implants supported by internal stents, and a delivery catheter to insert the implants into the body.






