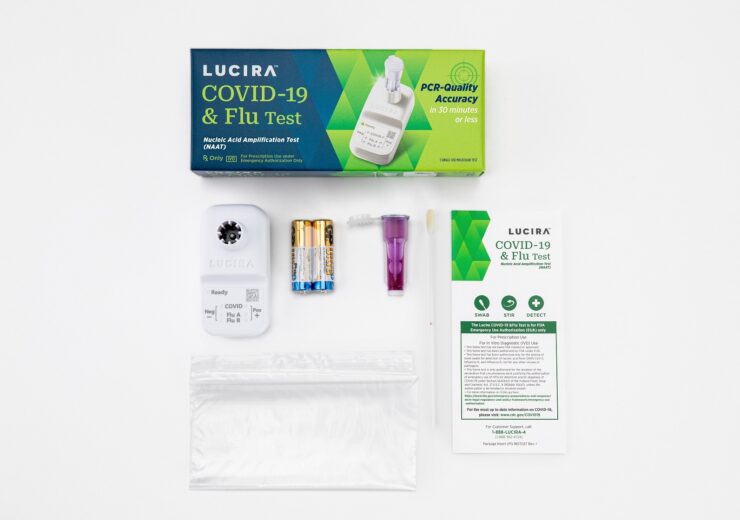
The Lucira Covid-19 & Flu test is a nucleic acid amplification test (NAAT)/molecular test, indicated for the independent diagnosis of Covid-19, Flu A, and Flu B, for use in point-of-care settings to deliver results within 11 to 30 minutes
Lucira Covid-19 & Flu test. (Credit: Lucira Health, Inc.)
US-based medical equipment manufacturer Lucira Health has secured the US Food and Drug Administration (FDA) emergency use authorisation (EUA) for its Lucira Covid-19 & Flu Test.
The Lucira Covid-19 & Flu test is a nucleic acid amplification test (NAAT)/molecular test, indicated for the independent diagnosis of Covid-19, Flu A, and Flu B.
Intended for point-of-care use, the test delivers results within 11 to 30 minutes, using a single shallow nasal swab and is available for use in CLIA-waived settings.
The company said that the combination test leverages the same platform and device design used in its FDA-authorised and commercialised Covid-19 test.
Lucira Health president and CEO Erik Engelson said “Covid-19 and flu viruses can both cause serious illness with similar symptoms, but each has unique prescription treatments that require diagnosis early in the infection to be effective.
“The inaccuracy of antigen Covid-19 tests makes such tests inadequate to use for early differential diagnosis of flu versus Covid-19.
“Clinicians can now facilitate simultaneous rapid testing at a mass scale to get patients on the path to recovery quickly. With this test and future testing capabilities, we aim to be at the forefront of quality, at-home health management.”
According to the company, its Lucira Covid-19 & Flu test has shown a similar performance compared to high-sensitive lab-based PCR assays in clinical trials.
The PCR-quality test, designed to fits inside the palm of a hand, runs on two AA batteries and comes with one shallow nasal swab to provide a positive or negative result.
The single-use test kit contains everything required to perform a test, eliminating the need to separately purchase and maintain any reader or instrument.
Furthermore, it is the first combination test among its portfolio of multiple assay tests that leverage Lucira technology platform’s ability to multiplex from a single sample, said Lucira.
In August this year, Health Canada authorised Lucira’s at-home Covid-19 and Influenza (Flu) test, under Interim Order for emergency use and commercialisation in Canada.
