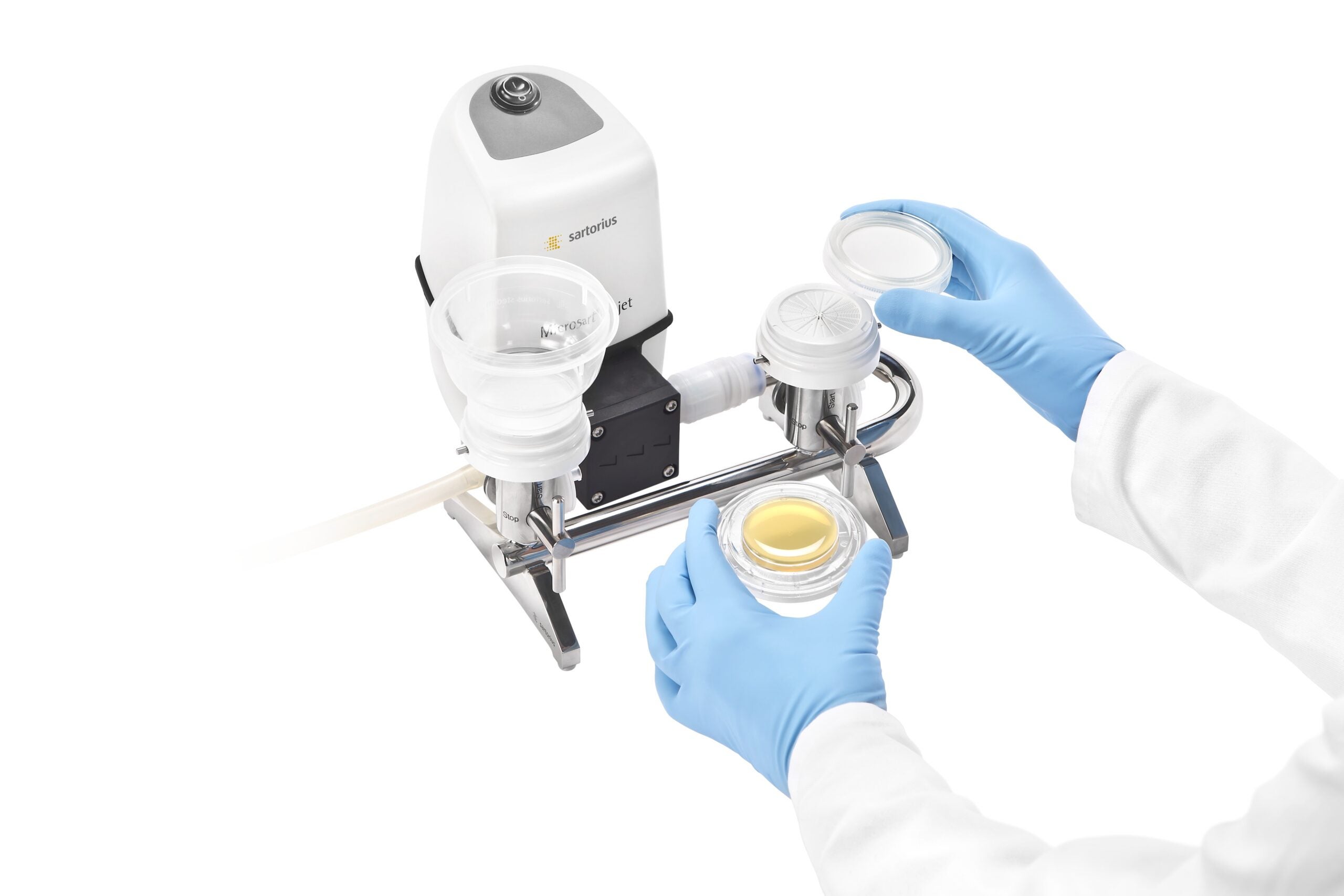
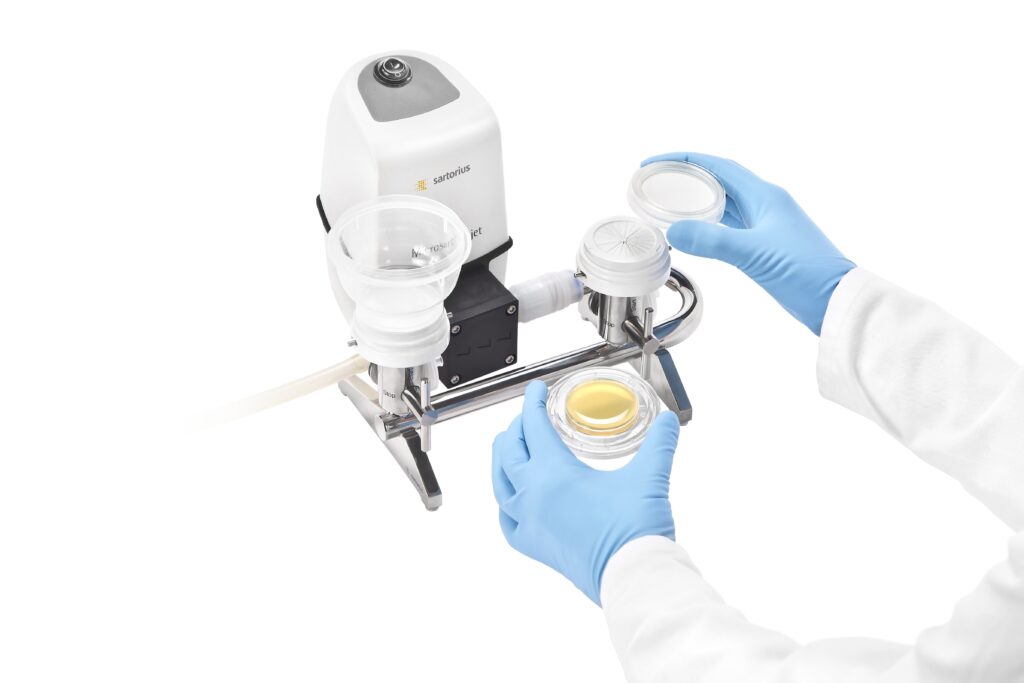
Endotoxins can cause a risk to patient safety. Even a low endotoxin concentration in the blood stream can produce inflammation in the human body; therefore, preventing endotoxin exposure to your patients is important.
To prevent patient exposure to endotoxins, frequent and reliable testing for endotoxin levels on medical devices is required. The LAL method, is one such reliable test method used to guarantee patient safety and the risk-free use of medical devices. An alternative method is the recombinant Factor C (rFC) assay that is completely animal-free. Sartorius enables you to get consistently reliable endotoxin testing results with purity-certified pipette tips and smart pipetting solutions.

